Percutaneous Transthoracic Needle
Biopsy:
Technique:
Prior to performing the procedure, patient consent must be obtained. As part of the preliminary work-up prior to the procedure, patients should have a prothrombin time (PT) and platelet count performed within two weeks of the procedure. Anticoagulation and antiplatelet medications (nonsteroidal anti-inflammatory agents) should ideally be discontinued for 7 days. Patients with a single lung (contralateral pneumonectomy) are not generally considered candidates for percutaneous biopsy procedures [22], although other authors suggest that biopsy of such patients can be performed with appropriate surgical backup, although at extremely high risk [12,32]. Some relative contraindications to transthoracic needle biopsy include an inability of the patient to cooperate (ie: cannot hold their breath or cannot be positioned for the procedure), underlying coagulopathy (PT > 15 sec, international normalized ratio > 1.3, or platelet count below 50,000/cm3), severe COPD (ie: patients who could not tolerate even a small pneumothorax), patient on mechanical ventilation (increases risk for pneumothorax and brochovenous fistula [8]), bullae in the vicinity of the the lesion to be biopsied, a vascular lesion (AVM or aneurysm), and pulmonary artery hypertension [1]. Pulmonary artery hypertension is a contraindication to biopsy of lesions located deep in the lung, and biopsy should only be performed when the lesion is peripheral and the PAH well controlled [25]. Aspirin use or chronic renal failure can cause platelet dysfunction not reflected in clotting profiles. Some authors [8] consider the previously described conditions to be contraindications to transthoracic biopsy- individual physicians should use sound clinical judgment and discuss the case with the appropriate specialists when making decisions regarding complicated patients.
An intravenous line, blood pressure monitor, electrocardiogram leads, and an oxygen saturation monitor should be placed. The biopsy room should be equiped with oxygen, suction, oral and nasal airways, an Ambu-bag (Ambu International, Glostrup, Denmark), a Pleur-evac device (Atrium Medical, Hudson, NH), and a crash cart. After lesion localization, the biopsy needle can be introduced alone, or as a coaxial system. The needle should be passed over the rib (not under) to avoid the neurovascular bundle. Fissures should be avoided because their transgression leads to three visceral pleural punctures rather than one. The use of thin (1-2 mm) localizing images may be very helpful in detecting blebs not seen on thicker section images, particularly in patients with underlying emphysema [8]. Specimens should be obtained during suspended respiration. Cytopathology support during the procedure is essential. In addition to aspiration samples, some centers perform core biopsies cutting needle.
Results:
Percutaneous needle biopsy can be
of tremendous value in patient diagnosis. With the use of 20 to
22 gauge aspiration needles and expert cytopathology,
sensitivities of 86 to 95% can be expected in the diagnosis of
primary intrathoracic malignancy.
For smaller nodules (5-7 mm) the sensitivity is lower (about
50%) [26]. However, higher diagnostic accuracy for small lesions
(≤ 1cm) has been reported with the use of fluoroscopic guided
biopsy (the disadvantage of CT fluoro
is the associated higher radiation exposure) [31]. Lower
sensitivities have also been reported for FNA of ground glass
and subsolid nodules [30]. In one
study, the diagnostic yield was as low as 35% for predominantly
ground glass nodules less than 10 mm in size [30]. The ability
to distinguish non-small cell from small cell carcinoma
approaches 100%. The sensitivity for metastatic disease is
between 85-90%. The accuracy of percutaneous
biopsy, however, is affected by the size of the lesion, and
decreasing accuracy is found to be associated with smaller
lesions [20,23]. Although, other
authors report excellent sensitivity even for nodules smaller
than 1 cm, particularly when core biopsy is used [36].
Previously, reliability in the diagnosis of benign lesions was not as high with a very variable yield of only 16 to 68% [1]. However, a core biopsy will often help to confirm that a lesion is benign. Core biopsy has also been shown to have a high sensitivity (92%) and specificity (90%) for biopsy of ground-glass nodules [27]. However, the biopsy results are more frequently non-diagnostic when the lesion is smaller than 2 cm or has a greater than 90% ground-glass component [27]. An automatic biopsy device with a short throw (1 cm) can significantly increase the diagnostic accuracy for benign and small lesions, while maintaining acceptable complication rates [2,10,11, 21]. Up to 76% of benign lesions can be accurately identified with the use of a core specimen [10]. In the diagnosis of lymphoma, core biopsy specimens can provide sufficient diagnostic material to guide therapy in 60 to 95% of cases. Unfortunately, the likelihood for an accurate diagnosis decreases with decreasing lesion size, even with the use of a automatic biopsy device [15] and there is also a higher incidence of non-diagnostic biopsies with subsolid nodules [38].. Cutting guns should never be used in a chronically infected cavity or in regions of bronchiectasis that might be associated with substantial bronchial artery hypetrophy as there is an increased risk for hemorrhage [8]. It should also be noted that when a biopsy gun fires, the shock wave distal to the biopsy needle tip is strong [28]. The arteries lateral to the needle will also be affected by the vibration [28]. The regions distal to the tip and lateral to the needle should be considered danger zones and major vessels should be well away from these areas [28].
Because of the low predictive value of a negative or non-diagnostic biopsy (up to 21% of nodules classified as nonspecific benignities [pathologic exam favoring, but not diagnostic of a benign cause] ultimately prove to be malignant [38]) one may ask does transthoracic needle biopsy ready aid in the management of patient's with solitary pulmonary nodules? It would seem that both patients with biopsies positive for malignancy and those which are negative may still require thoracotomy. However, a non-diagnostic biopsy containing atypical cells is associated with a much higher risk for underlying malignancy (up to 90%) and should therefore undergo rebiopsy or excision [38]. Lesions with insufficient specimens can have a malignancy rate up to almost 47% [38]. For suspicious nodules, video assisted thoracoscopic excision can be used for complete removal of the nodule if it is in an accessible location. Tumor seeding along the incisional tract has been reported, and specimens suspected of being malignant should be removed in some type of receptacle [3].
Percutaneous needle aspiration may also be of benefit in the diagnosis of pulmonary infection in immunocompromised patients [14].
Complications:
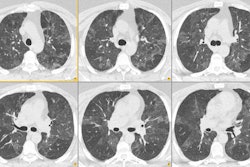
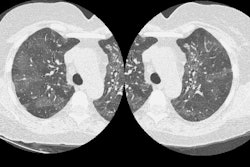
1. Pneumothorax: The incidence of pneumothorax following CT guided transthoracic needle biopsy is generally reported to be between 20 to 30% and is felt to be somewhat higher than the incidence of pneumothorax following fluoroscopic guided biopsy. Biopsy of small nodules (less than 1 cm) is also associated with an increased incidence of pneumothorax (about 60%) and an increased likelihood for chest tube placement (up to 31% of patients that develop pneumothorax) [20]. Most pneumothoraces occur during or within the first hour after biopsy. Only about 2% of pneumothoraces will first appear on four hour post-procedure films. Factors which are associated with an increased risk of pneumothorax include emphysematous changes within the lungs, coughing or breathing during the procedure, increased lesion depth (when traversing aerated lung or crossing fissures), smaller lesion size, multiple pleural punctures, using cutting needles or biopsy guns to obtain core biopsies, using a larger gauge needle for the biopsy [24], prone or lateral patient positioning [12,17], shallow pleural puncture (especially under 45-50 degrees- possibly due to a larger or elongated pleural hole) [17,31], and positive pressure ventilation. The use of a coaxial technique permits multiple needle passes from a single pleural puncture. The use of spring loaded biopsy guns should be reserved for large lesions with a diameter exceeding the throw of the needle (usually 20-23 mm) or for those which have a broad pleural attachment. Whether to treat the pneumothorax with a chest tube depends on the patients pulmonary reserve, if the patient is symptomatic (dyspnic), or whether the pneumothorax is enlarging over time. Chest tube drainage of post-procedure pneumothorax is required for between 2% to 14% of cases [4,25,31]. The severity of the pneumothorax and the need for chest tube placement appear to be correlated with the severity of underlying obstructive airway disease (emphysema) [5,13,31]. The majority of pneumothoraces which require chest tube drainage are identified immediately following the procedure [18].
In an attempt to reduce the prevalence of pneumothorax
following the procedure several maneuvers have been suggested.
Positioning the patient so that the biopsy side is in the
dependent position is perhaps the most successful [39]. It is
postulated that this maneuver reduces both alveolar size and the
differential of alveolar and pleural pressure. These changes
favor the development of dependent atelectasis
and close approximation of the parietal and visceral pleura. In
one study, PTX occured in about 11% of patients with biopsy-side
down positioning, as compare to 27% for patients either prone or
supine [39]. Hemoptysis was also less frequent in the
biopsy-side down patients (5% vs 10%) [39].
The administration of oxygen (100% at 2-3 L/min) to patients
before, during, and following the procedure may also be useful.
The theoretic basis for this is that should a pneumothorax develop while the patient
is breathing high concentrations of oxygen, the air in the
pleural space would also be oxygen rich. A pneumothorax
will decrease more rapidly in this setting since oxygen is resorbed into blood faster than air.
Aspiration of large immediate post-procedure pneumothoraces using a standard 18 g IV
catheter may decrease the need for chest tube placement. In one
study of patients with large post-procedure pneumothoraces, immediate aspiration of
the air avoided the need for chest tube placement in 70% of
these patients [6]. Aspiration followed by intrapleural blood
patching (using 15 mL of autologous blood) has been shown to
further decrease the need for subsequent chest tube placement
[33,35].
Patients who develop clinically important pneumothoraces have also been managed as outpatients with short-term small caliber chest tubes [7]. Plugging the biopsy tract with autologous blood clot has been advocated by some authors to decrease the risk of pneumothorax, although results are variable [16].
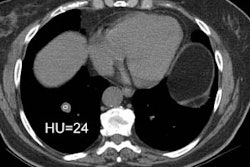
2. Hemorrhage/hemoptysis: The
incidence of hemorrhage following transthoracic
biopsy ranges from 1 to 41%- it is the second most common
complication of transthoracic
biopsy. Despite the frequency of hemorrhage, the need for
hospitalization is uncommon (less than 1% of cases in one study
[37]). The use of a biopsy gun is associated with an increased
risk for hemorrhage [8,11].
Hemorrhage is almost always self-limiting [1]. The likelihood of
hemorrhage is greater for vascular lesions (such as vascular
metastases or chronic inflammatory cavities [due to enlarged
bronchial arteries]), patients with an underlying bleeding
diathesis (thrombocytopenia, aspirin use), patients with
pulmonary arterial or venous hypertension, smaller and non
subpleural lesions, subsolid nodules, female patients, older
patients, underlying emphysema, coaxial biopsy technique,
and with the use of larger cutting needles [12,37]. Patients
with hemorrhage should be placed biopsy side down to decrease
the risk of blood being carrying into the opposite lung. During
parasternal biopsy procedures, care
should be taken to avoid the internal mammary vessels which lie
about 1.25 cm lateral to the lateral margin of the sternum.
3. Systemic air embolization: This is a very rare complication of the procedure- incidence 0.07-0.21% [19,29]. Air embolism most likely occurs due to air entering the pulmonary vein either directly from the needle open to the atmosphere or from a broncho- or alveolovenous fistula induced during placement of the needle. Factors which increase airway pressure result in an increased risk for air embolism including coughing, the deep inspiration which precedes coughing, and positive pressure ventilation. Biopsy guns may also increase the risk for air embolism [8,12]. Complications of air embolism include myocardial infarction, stroke, or death. Other factors that may be associated with an increased risk for air embolism include use of a large bore biopsy needle (19 gauge or larger) and biopsy through disease lung [19]. As little as 0.5 ml of air is sufficient to induce coronary artery ischemia and fatal arrhythmias. Treatment consists of placing the patient in a left lateral decubitus position (to prevent air within the left atrium from embolizing systemically) or Trendelenberg position (to keep air out of the cerebral circulation). Ventilatory support with 100% oxygen should be administered to promote resorption of air bubbles. Transfer to a hyerbaric oxygen chamber may be necessary. [1,26]
4. Malignant seeding of the biopsy tract: Although seeding may occur, it is an extremely rare complication (0.012-0.56%) [29]. The risk malignant seeding of the biopsy track is random- no definite risk factors have been described [9].
5. Death: The mortality rate from transthoracic
needle biopsy is 0.02% to 0.15% [1,25]
Post-biopsy Management:
Some centers obtain post-biopsy chest radiographs immediately
following the procedure, while others delay this exam for one
hour to avoid straining associated with rising from a recumbent
to a seated or standing position. Selected CT images following
the procedure can also be used to evaluate for the presence of a
pneumothorax. Patients should be
monitored with vital signs, breath sounds, and oxygen
saturations every 15 minutes for one hour and every 30 minutes
thereafter. The biopsy site should be placed in a dependent
position following withdrawal of the biopsy needle for up to 3
hours [34]. This maneuver decreases alveolar volume adjacent to
the puncture site, uses the weight of the lung to help tamponade the puncture, and raises intrapleural pressure thereby decreasing
the amount of air leak. An additional benefit of this position
is the prevention of transbronchial
spread of hemorrhage that may complicate the procedure. A rapid
needle-out patient roll-over time has been suggested to result
in a decrease in the rate of pneumothorax (this is accomplished
by having the patient's stretcher besides the CT scanner prior
to needle removal) [34].
The use of a blood patch has been described and can decrease the rate of post-procedure pneumothorax (although it does not appear to work for larger needle biopsies [17 g])- the proposed mechanism of action is formation of a patch of clotted blood that adheres to the site of air leakage [33,35]. Patients should remain in the biopsy site dependent position for at least 2 hours. If patients that have no pneumothorax following the procedure and at 4 hours post biopsy can be discharged home. Patients with only a small pneumothorax identified on delayed chest radiographs can probably be discharged safely [18]. Patients should be instructed to avoid straining and all but mild physical exertion until the following day. Patients should spend the night following the procedure with a family member of friend [12]. Patients should be instructed to procede immediately to an emergency room for any pleuritic chest pain, hemoptysis (of more than a teaspoon of fresh blood), or shortness of breath. [1,12]
If a small or asymptomatic pneumothorax is present post-procedure CXR's should be obtained at 2 and 4 hours. If the pneumothorax remains stable and the patient remains asymptomatic, they may be released. If the pneumothorax is enlarging (15 to 35% pneumothorax- depending on the patients respiratory status) or becomes symptomatic, an 8 french catheter with a hollow inner trocar should be placed in the lung apex over the second intercostal space in the midclavicular line. The air in the pleural space should be aspirated and the catheter attached to a Heimlich valve or Pleurevac system. Most pneumothoraces requiring chest tube drainage are detected within one hour of the biopsy. [1]
REFERENCES:
(1) J Thorac Imag 1997; Klein JS, et al. Transthoracic needle biopsy: An overview. 12: 232-249
(3) Ann Thorac Surg 1995 Jan;59(1):42-45
(5) Radiology 1996; 198: p.371-375
(6) Radiology 1996; 200: 695-697
(7) Radiology 1997; 205: 249-252
(8) J Thorac Imag 1997;12: 259-271
(9) J Thorac Imag 1998; 13: 2-6
(11) Radiology 1998; Lucidarme O, et al. Intrapulmonary lesions: Percutaneous automated biopsy with a detachable, 18-gauge, coaxial cutting needle. 207: 759-765
(12) Radiology 1998; Moore EH. Technical aspects of needle aspiration lung biopsy: A personal perspective. 208: 303-318 (No abstract available)
(13) AJR 1999; Laurent F, et al. Pneumothoraces and chest tube placement after CT-guided transthoracic lung biopsy using a coaxial technique: Incidence and risk factors. 172: 1049-1053
(14) AJR 2000; Hwang SS, et al. The value of CT-guided percutaneous needle aspiration in immunocompromised patients with suspected pulmonary infection. 175: 235-238
(15) AJR 2000; Tsukada H, et al. Diagnostic accuracy of CT-guided automated needle biopsy of lung nodules. 175: 239-243
(16) Radiology 2000; Lang EK, et al. Autologous blood clot seal to prevent penumothorax at CT-guided lung biopsy. 216: 93-96
(17) Radiology 2001; Ko JP, et al. Factors influencing pneumothorax rate at lung biopsy: Are dwell time and angle of pleural puncture contributing factors? 218: 491-96
(18) Radiology 2001; Dennie CJ, et al. Transthoracic needle biospy of the lung: Results of early discharge in 506 outpatients. 219: 247-251
(19) AJR 2002; Arnold BW, Zwiebel WJ. Percutaneous transthoracic needle biopsy complicated by air embolism. 178: 1400-1402
(20) Radiology 2002; Wallace MJ, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (<1-cm) pulmonary lesions. 225: 823-828
(21) AJR 2003; Yamagami T, et al. Combining fine-needle aspiration and core biopsy under CT fluoroscopy guidance: a better way to treat patients with lung nodules? 180: 811-815
(22) Society of Thoracic Radiology Annual Meeting Syllabus 1997; 1-6
(23) AJR 2003; Ohno Y, et al. CT-guided transthoracic needle aspiration biopsy of small (< 20 mm) solitary pulmonary nodules. 180: 1665-1669
(24) Radiology 2003; Geraghty PR, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. 229; 475-481
(25) AJR 2005; Maher MM, et al. Percutaneous lung biopsy in a patient with a cavitating lung mass: indications, technique, and complications. 185: 989-994
(26) Radiology 2006; Winer-Muram HT. The solitary pulmonary nodule. 239: 34-49
(27) AJR 2008; Kim TJ, et al. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. 190: 234-239
(28) AJR 2009; Tsai I-C, et al. CT-guided core biopsy of lung lesions: a primer. 193: 1228-1235
(29) AJR 2009; Ibukuro K, et al. Air embolism and needle track implantation complicating CT-guided percutaneous thoracic biopsy: single institution experience. 193: 1453
(30) Radiology 2009; Godoy MCB, Naidich DP. Subsolid pulmonary nodules and the spectrum of peripheral adenocarcinomas of the lung: recommended interim guidelines for assessment and management. 253: 606-622
(31) AJR 2010; Hiraki T, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. 194: 809-814
(32) AJR 2011; Cronin CG, et al. Percutaneous
lung biopsy after pneumonectomy:
factors for improving success in the care of patients at high
risk. 929-934
(33) AJR 2011; Wagner JM, et al. CT-guided lung biopsies:
pleural blood patching rduces the rate of chest tube placement
for postbiopsy pneumothorax. 197: 783-788
(34) Radiology 2012; O'Neill AC, et al. Rapid needle-out
patient-rollover time after percutaneous CT-guided transthoracic
biopsy of lung nodules: effect on pneumothorax rate. 262:
314-319
(35) AJR 2013; Malone LJ, et al. Effect of intraparenchymal
blood patch on rates of pneumothorac and pneumothorax requiring
chest tube placement after percutaneous lung biopsy. 200:
1238-1243
(36) AJR 2013; Choi SH, et al. Percutaneous CT-guided
aspiration and core biopsy of pulmonary nodules smaller than 1
cm: analysis of outcomes of 305 procedures from a tertiary
referral center. 201: 964-970
(37) Radiology 2016; Tai R, et al. Frequency and severity of
pulmonary hemorrhage in patients undergoing percutaneous
CT-guided transthoracic lung biopsy: single-institution
experience of 1175 cases. 279: 287-296
(38) Radiology 2019; Kyung KH, et al. Nondiagnostic
percutaneous transthoracic needle biopsy of lung lesions: a
multicenter study of malignancy risk. 290: 814-823
(39) Radiology 2019; Drumm O, et al. CT-guided lung biopsy:
effects of biopsy-side down position on pneumothorax and chest
tube placement. 292: 190-196