Meconium Aspiration Syndrome (MAS):
View cases of meconium aspiration
Clinical:
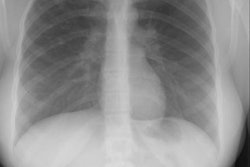
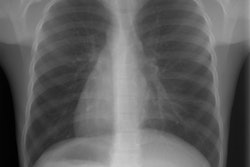
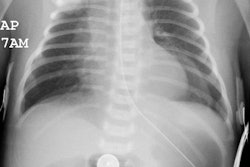
Meconium aspiration produces bronchial obstruction and a chemical pneumonitis. Meconium aspiration is the most common cause of significant morbidity and mortality among full- and post term infants [1]. Aspirated meconium causes a chemical pneumonitis and inactivates surfactant [1]. The resultant hypoxia and vasoconstriction of the peripheral pulmonary arterioles can lead to pulmonary hypertension and right to left shunting across a PDA or patent foramen ovale. MAS also predisposes to infection [1].The disorder occurs in conditions of fetal distress (often post mature infants) due to stress induced defecation and in utero gasping due to hypoxia. Meconium staining of the amniotic fluid can be seen in 10-15% of births, by MAS occurs in only about 5% of these cases [1]. There is often a rapid clinical improvement, but x-ray changes may be slow to clear. Complications include air-block with subsequent pneumo-thorax/ mediastinum (in 25%) and infection (meconium is an excellent culture medium). Morbidity is typically the result of anoxic brain injury. Differential considerations include wet lung (transient tachypnea of the newborn), or neonatal viral pneumonia.
X-ray:
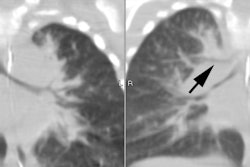
On CXR there are patchy, asymmetric opacifications which represent areas of atelectasis or pneumonitis and coarse, irregular pulmonary densities associated with HYPERINFLATION. There may occasionally be small effusions (up to 20%). Also look for pneumothorax (in 10-40% of cases) or pneumomediastinum. Hyperinflation in the neonate is indicated by the level of the hemidiaphragms which will appear straight or flattened on the lateral view.REFERENCES:
(1) AJR 2018; Liszewski MC, Lee EY. Neonatal lung disorders: pattern re to diagnosis. 210: 964-975