Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy:
View cases of arrhythmogenic right ventricular dysplasia
Clinical:
RV myocardial fat is frequently seen in the non-disease heart
during autopsy and the frequency and degree of myocardial fat
increase with age [11]. The RV myocardial fat is usually confined
to the outer half of the RV wall [11]. The prevalence of RV
myocardial fat at CT has been reported to be 16-43%- on axial CT
images it is most frequently located in the basal superior wall,
followed by the middle superior wall and then the RVOT (the basal
superior and middle superior walls on axial CT correspond to the
anterolateral RV free wall on short-axis CT images) [11]. As the
amount of fatty infiltration increases, the RV myocardium
maintains its normal thickness or thickens [11]. Other conditions
associated with fatty RV infiltration in otherwise healthy
subjects include obese individuals and long-term steroid users
[12].
Arrhythmogenic right ventricular dysplasia (ARVD) is a rare (one in 10,000 individuals in the US [10]; prevalence 0.05-0.1% [14]) cardiomyopathy of unknown etiology that is characterized by thinning and partial/segmental or total fibrofatty replacement of the right ventricular myocardium, ventricular tachycardia originating in the right ventricle, and regional and global right ventricular contractile abnormalities [12]. Histologically, there is apoptosis of myocytes that are replaced by fibrofatty tissue [10]. Most affected patients have localized or patchy areas of segmental right ventricular thinning and akinesia or dyskinesia. The disease often affects the RV outflow tract, the base of the RV (the inferior or diaphragmatic wall beneath the posterior leaflet of the tricuspid valve), and the RV apex (collectively termed the triangle of dysplasia) [10,11]. In nearly 50% of cases, saccular aneurysms are visible at the apex and inferior wall [11]. Fibrofatty replacement may also occur in the LV (47-76% of cases) and in the interventricular septum (20% of cases) [11].
Patients are usually males (3:1) [2], although other authors suggest equal prevalence in males and females [14]. Patients typically have minimal symptoms- symptoms, when present, frequently occur with exercise [2]. The condition is a major cause of sudden death in young adolescents (in the US ARVD accounts for 5% of the sudden cardiac deaths in individuals younger than 35 years and in 20-50% of csaes, sudden cardiac death is the first manifestation of the disorder) [3,10,14]. The disorder appears to be inherited with a strong familial tendency (possibly an autosomal dominant disorder with variable expression and penetrance) [3,14]. Most patients are between the ages of 20 to 50 years. The typical clinical manifestation consists of ventricular arrhythmias with a left bundle branch pattern [3]. The ECG frequently shows T-wave inversion in the anterior precordial leads. Rarely, post-excitation waves are recorded in the ST segment (epsilon waves- representing delayed depolarization of some parts of the right ventricle) [2]. Other ECG findings include right ventricular parietal block and reduced QRS amplitude [16]. The death rate for patients with ARVD has been estimated to be 2.5% per year [3]. The disease must be differentiated from right ventricular outflow tract tachycardia- which has a lower risk of sudden death [2].
Unfortunately, myocardial biopsy may fail to confirm the diagnosis due to the segmental nature of the lesion [3]. Even when the biopsy specimen is obtained from the right ventricular free wall, the sensitivity is 67% and the specificity 92% [3]. It is also important to remember that fatty infiltration of the right ventricle can be seen in more than 50% of normal elderly patients [3].
The diagnosis of ARVD is based on the presence of alterations of
RV morphology and function at 2D echo or MR and include fibrofatty
replacement of the myocardium at endomyocardial biopsy,
repolarization abnormalities on ECG, depolarization or conduction
abnormalities on ECG, ventricular arrhythmias, and a family
history or ARVD or sudden cardiac death before age 35 years [13].
Criteria for the diagnosis are classified as major or minor- to
establish the diagnosis requires two major criteria, one major
plus two minor criteria, or four minor criteria [5]. Minor
criteria include a family history of premature sudden cardiac
death (under age 35 years) or suspected ARVD, ECG abnormalities in
the right precordial leads (V1-V3), and mild global or segmental
right ventricular wall motion abnormalities [5]. Major criteria
include family disease confirmed at necropsy or surgery, epislon
waves on ECG, severe segmental or global right ventricular
dilatation, right ventricular aneurysms, and fibrofatty
replacement of right ventricular myocardium [5]. See also the 2010 Task Force
Criteria.
Treatment consists of antiarrhythmic agents, catheter ablation (for localized disease), implantable cardioverter defibrillators, and surgery (ventriculotomy with total disconnection of the right ventricular free wall) [3].
A more benign form of ARVD termed fatty replacement of the right ventricle has also been described [4]. Unlike ARVD, the right ventricular wall is normal in size or thickened, and does not show myocyte atrophy or inflammation [4]. Arrhythmias are far less common in this condition [4].
X-ray:
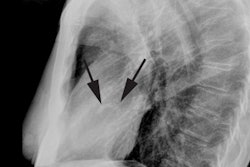
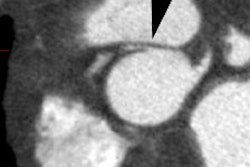
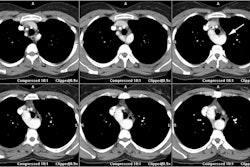
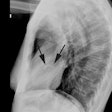
CT: Characteristic findings on CT include dilatation of the RV, abundant epicardial fat, myocardial fat in the RV trabeculae and moderator band, conspicuous trabecule, and scalloped or bulging appearance of the RV free wall [11]. Remember- in physiologic fatty infiltration of the RV, the wall remains normal in thickness, were as the wall is usually almost inperceptibly thin in ARVD [11].
MRI: The MR technique is focused on visualization of the free wall of the right ventricle. This is best accomplished by using a surface coil with the patient lying prone on the coil. The ECG-gated, short-TE images are acquired by using a 256-192 matrix with two averages and a small field of view in order to maximize spatial resolution. Flow signal artifact, displaced in the phase-encoding axis, can produce factitious bright signal foci in the free wall of the right ventricle. Double saturation bands are placed behind the free wall to suppress flow artifact from the ventricular blood pool [2]. A saturation band over the left ventricle can help to decrease pulsation artifact. Better images can also be obtained by turning off the signal from the posterior elements of the phased array coil as information from this region is not required to assess the RV free wall.
Normal RV thickness is between 2-4 mm. In ARVD the examination demonstrates dilatation of the RV, thinning of the RV free wall (<2mm) in regions of fibrosis (especially focal thinning in the lower infundibular region in early disease), moderator band hypertrophy [12], and increased signal intensity on T1 images in regions which have been replaced by fat. Fat infiltration can be confirmed with the use of fat-saturation sequences, but is often difficult to detect on MR imaging. The presence of fatty infiltration is associated with a greater likelihood of inducible ventricular tachycardia (33% of patients with increased signal, as opposed to 16% of patients without this finding). However, the presence of fat in the RV wall can lead to a false positive diagnosis as fatty deposits can occur in a wide variety of conditions [8]. LV involvement may be seen at MR- typically the epicardium in the infero-posterior LV [10].
Cardiac MR assessment of ARVD is now based primarily on functional and volume abnormalities [12]. Cine MR images will demonstrate poor RV contractile function and dyskinesis of the affected segment(s). The site of involvement is characteristically found in the inferior subtricuspid area, the RV apex, and the RV infundibulum- also referred to as the "triangle of dysplasia" [12]. RV volumes are also typically augmented (end-diastolic volume >150 mL and end-systolic volume > 70 mL) [9]. Fat in the atrioventricular groove or near the anteroapical portions of the RV is epicardial and should not be confused with intramural fat [2].
Delayed hyperenhancement may be seen in up to 67% of patients on post contrast imaging in the regions of fibrofatty infiltration- particularly the outflow tract and anterobasal region [6,7]. Other authors indicate that the inferolateral wall is most commonly affected with subepicardial and midwall delayed enhancement (the inferoseptal junction, anterolateral wall, and anterior wall can also be affected) [8]. The extent of delayed enhancement correlates with inducibility of ventricular arrhythmias during electrophysiologic testing [10].
REFERENCES:
(1) Magn Reson Imaging Clin N Am 1996; May 4(2): 307-325
(2) AJR 1994; Blake LM, et al. MR features of arrhythmogenic right ventricular dysplasia. 162: 809-812
(3) Radiographics 2002; Kayser HWM, et al. Diagnosis of arrhythmogenic right ventricular dysplasia: a review. 22: 639-650
(4) Radiographics 2002; Gaerte SC, et al. Fat-containing lesions of the chest. 22: S61-S78
(5) AJR 2004; Abbara S, et al. Value of fat suppression in the MRI evaluation of suspected arrhythmogenic right ventricular dysplasia. 182: 587-591
(6) AJR 2007; Lim RP, et al. Non-ischemic causes of delayed myocardial hyperenhancement on MRI. 188: 1675-1681
(7) Radiographics 2006; Vogel-Claussen J, et al. Delayed enhancement MR imaging: utility in myocardial assessement. 26: 795-810
(8) J Nucl Cardiol 2008; O'Hanlon R, et al. Evaluation of non-ischemic cardiomyopathy using cardiovascular magnetic resonance. 15: 400-416
(9) AJR 2008; Belloni E, et al. MRI of cardiomyopathy. 191: 1702-1710
(10) J Nucl Cardiol 2010; Jonnalagadda N, et al. Role of cardiac imaging evaluation of patients with documented or suspected ventricular arrhythmias. 17: 145-152
(11) Radiographics 2010; Kimura F, et al. Myocardial fat at
cardiac imaging: how can we differentiate pathologic from
physiologic fatty infiltration. 30: 1587-1602
(12) Radiology 2012; O'Donnell DH, et al. Cardiac MR imaging of
nonischemic cardiomyopathies: imaging protocols and spectra of
appearances. 262: 403-422
(13) Radiographics 2013; Stojanovska J, et al. Congenital and
hereditary causes of sudden cardiac death in young adults:
dianosis, differential diagnosis, and risk stratification. 33:
1977-2011
(14) Radiographics 2014; Rastegar N, et al. Cardiac MR findings
and potential diagnostic pitfalls in patients evaluated for
arrhythmogenic right ventricular cardiomyopathy. 34: 1553-1570
(15) Radiographics 2017; Hashimura H, et al.
Radiologic-pathologic correlation of primary and secondary
cardiomyopathies: MR imaging and histopathologic findings in
hearts from autopsy and transplantation. 37: 719-736
(16) J Nucl Cardiol 2017; Rachoin R, et al. Arrhythmogenic
ventricular cardiomyopathy and sudden cardiac death: left or
right? 24: 527-533