ANCA-associated granulomatosis
Granulomatosis with Polyangiitis
Previously Wegener's Granulomatosis
Clinical:
Wegener's (now referred to as Granulomatosis with polyangiitis or ANCA-associated granulomatosis [14]) is a necrotizing granulomatous process with destructive angiitis that involves small and medium sized arteries and veins. It may be an immunologic (autoimmune) disorder, but an etiology has not yet been identified. There is no relationship to smoking [7]. Wegener's is part of the spectrum of vasculitic disease which include polyarteritis nodosa, allergic granulomatosis, and hypersensitivity angiitis. In Wegener's there is a systemic necrotizing capillitis/ vasculitis consisting of:
1- A granulomatous vasculitis of the upper (nasal, oral, or sinus inflammation) and lower respiratory tracts which are involved in over 90% of cases. Rhinosinus involvement may lead to cartilage destruction and a "saddle nose" deformity [16]. Tracheobronchial tree involvement is common (25-60% of patients) and may result in tracheal stenosis/stricture (in about 7% of patients [11], most commonly in the subglottic region [11]), bronchial stenosis, or ulcerating tracheobronchitis and resulting in stridor, dyspnea, and postobstructive pneumonia [13]. Lung involvement develops in 60-85% of patients [16,17]. Isolated pulmonary involvement is referred to as "limited Wegeners", is associated with the best prognosis, and affects women slightly more frequently than men.
2- A necrotizing glomerulonephritis: Although only about 40% of patients have renal involvement at presentation, 80% to 90% ultimately develop renal disease [15]. Rarely the renal disease may manifest as renal masses [10].
3- A systemic small vessel vasculitis involving numerous organ systems: joints (67%), skin (45%), muscle, eyes (58%), middle ear (61%), heart (12%), and the CNS. [7]
The disorder can occur at any age, but peaks in 40-50's and is uncommon in children (the median age of onset is 45 years [14]). There is a slight male predominance (2:1), but other authors suggest that males and females are affected equally [15]. The majority of cases (90%) occur in white patients [15]. Patients can complain of URI-like symptoms with dry cough (78%), fever (83%), malaise, and weight loss, but they also experience hemoptysis (40%), dyspnea (generally mild), pleuritic pain (30%), and sinusitis with epistaxis. Alveolar hemorrhagic syndrome is characterized by dyspnea, hemoptysis (with blood originating from the distal airways by bronchoscopy), severe anemia, and diffuse bilateral alveolar infiltrates on CXR. Wegener's and microscopic polyangiitis are the most common causes of diffuse alveolar hemorrhage- representing 41% of cases [13]. Bronchoscopy is abnormal in just over 50% of cases- demonstrating bronchial stenosis, ulcerations, or inflammatory lesions. Bronchoalveolar lavage fluid in patients with Wegener's demonstrates a marked increase in neutrophils (mean 22-42%) and an increase in eosinophils can also be seen (mean 2.6-4%). BAL fluid will also be ANCA positive [3].
Anti-neutrophilic cytoplasmic antibodies (ANCA) are autoantibodies directed against constituents of polymorphonuclear leukocytes (neutrophils) and monocytes. These autoantibodies are divided into those which have a cytoplasmic staining pattern (C-ANCA) and those with a perinuclear staining pattern (P-ANCA). About 90-96% of patients with Wegener's (and 67-75% of patients with limited disease) are ANCA positive. C-ANCA predominates and it is present in more than 75% of patients with active Wegner's. The C-ANCA antibodies are usually directed against proteinase 3 [15]. The specificity of ANCA for Wegener's is 98% and titers correlate with disease activity (ie: a rise in C-ANCA titer usually portends a clinical exacerbation). Positive ANCA levels are occasionally associated with other arteritidies / vasculitidies. Most patients with polyarteritis nodosa and about 50% of patients with Churg-Strauss sydrome are ANCA positive, although the P-ANCA predominates. HIV infection has also be associated with a positive ANCA.
Extra-pulmonary features of Wegener's include sinusitis, rhinitis, otitis, and epistaxis. The glomerulonephritis results in hematuria (up to 65% of cases). Joint and muscular involvement can produce a migratory polyarthropathy (up to 34% of cases) or myalgias, respectively. Mucocutaneous involvement can result in macules, papules, cutaneous nodules, or oral ulcerations. Ocular involvement can result in conjunctivitis and episcleritis. Peripheral neuropathy can occur in 10%-21% of patients [8]. Cerebral and meningeal involvement occurs in 2%-8% of cases [8].
In the absence of therapy, about 90% of patients die within 2
years [15]. For limited disease localized to the upper airways,
treatment with corticosteroids, methotrexate, or azathioprine may
be adequate [15]. Treatment for more advanced disease consists of
immunosuppressive therapy with cyclophosphamide (associated with
an increased risk for bladder cancer), either with or without
steroids, and there is a 70-90% long term remission rate with this
therapy [2,14]. Treatment generally results in rapid clearing of
parenchymal lung abnormalities without significant residual
pulmonary impairment, but the imaging findings may lag behind
clinical improvement [12].
A poor prognosis is associated with the presence of diffuse alveolar hemorrhage, severe azotemia, advanced age, and proteinase 3 ANCA positivity [14].
X-ray:
CXR generally demonstrates bilateral (82%) or unilateral (18%), variable sized, well (most commonly) or poorly defined parenchymal nodules with a lower lobe predominance. The apices are typically spared. The number of nodules is variable, but is generally less than 10. The nodules frequently cavitate and typically have a thick, shaggy wall. Fluid levels within the nodules are uncommon.
On CT, lung nodules/masses are the most common finding and are seen in 40-90% of patients [12,13,14]. The nodules are usually multiple, bilateral, 2-4 cm in size, usually random in distribution, subpleural (89% of patients), or peribronchovascular (41% of patients), and lack a zonal predilection [1,12,14,15]. Feeding vessels entering the nodular lesions are very commonly identified at CT and reflect the angiocentric nature of the disorder [7]. A CT "halo sign" (a rim of ground glass attenuation surrounding the nodule) may be seen due to surrounding hemorrhage in up to 15% of cases [7,12,13]. The "atoll sign" - a rounded area of consolidation with contral ground-glass attenuation- can also be seen [15]. Cavitation occurs in approximately 25% of nodules larger than 2 cm [12]- typically thick walled with an irregular inner margin [13] (other authors suggest cavitation can occur in up to 50% of nodules larger than 2 cm [15]). Nodules may regress and reappear spontaneously. With treatment, approximately 50% of the nodules resolve, 40% diminish in size, but leave residual damage, and 10% remain unchanged [13]. Pleural effusions are noted in 5-50% of cases, typically the result of pleural involvement.
|
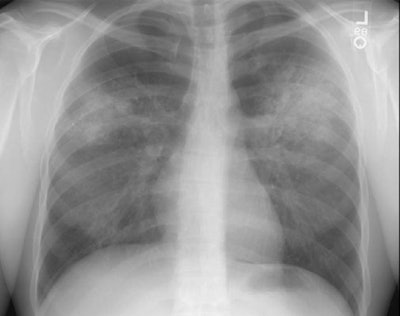
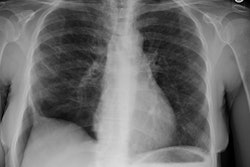
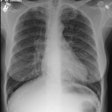
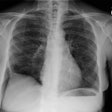
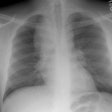
Wegeners: The patient shown in the case below presented with hemoptysis. CXR demonstrated cavitary areas of consolidation in the right mid and left upper lungs (Click CXR to enlarge image). Chest CT revealed cavitary nodules, cavitary areas of consolidation, and non-cavitary areas of patchy parenchymal consolidation. Patient was C-ANCA positive and lung biopsy revealed Wegener's. |
 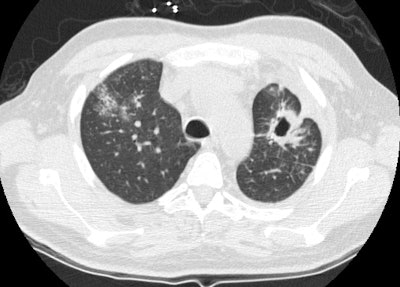 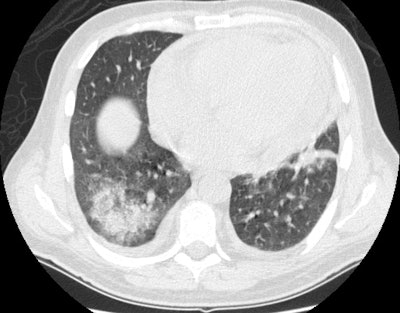 |
Patchy focal or diffuse parenchymal consolidations and ground
glass opacities are found in 30-53% of cases and are usually due
to pulmonary hemorrhage. The opacities are generally random
or patchy in distribution [14], but bilateral perihilar and
peribronchovascular distributions are the most common [15]. The
infiltrates may be found to be cavitary by computed tomography.
Diffuse alveolar hemorrhage occurs in about 10% of patients
producing a diffuse ground-glass opacity that often spares the
subpleural lung [12,13].
Up to 10% of patients have centrilobular tree-in-bud nodules that
may be secondary to bronchiolar wall involvement or retained blood
in the distal airways [17].
Hilar and mediastinal adenopathy is RARE and not considered a feature of the disorder. Less common manifestations include endobronchial disease which can result in atelectasis.
|
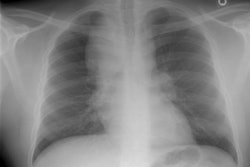
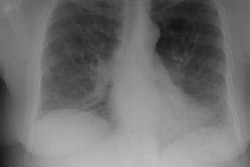
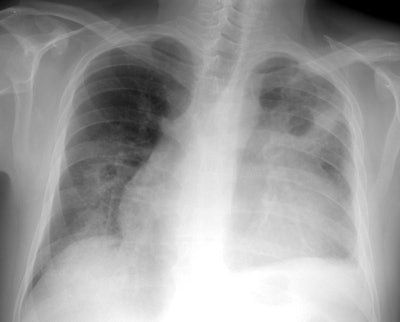
Wegeners overlap syndrome with extensive pulmonary hemorrhage: The patient shown in the image below had an extensive travel history and his parents were missionaries in New Guinea. He was referred to the rheumatology service for complaints of polyarthralgia. On exam, additional history revealed complaints of epistaxis, and later hemoptysis. CXR was obtained and demonstrated bilateral consolidations which were primarily confined to the superior segments of the lower lobes and posterior upper lobe segments. A PPD was placed and was negative, and the patient was HIV negative. Although plain films of the paranasal sinuses were normal, on direct visualization, mucosal ulcerations were identified. At bronchoscopy, blood was noted within the airways- indicating that the parenchymal abnormalities were likely the result of hemorrhage. Both serum and BAL fluid were C-ANCA positive and a diagnosis of Wegener's was made. However, the open lung biopsy specimen demonstrated both features of Wegener's and an eosinophoilic vasculitis (Churg-Strauss) and the final diagnosis was that of an overlap vasculitis. The patient was treated with steroids and methotrexate with a rapid clearing of the parenchymal abnormalities. |
|
|
Airway involvement is a late complication of the disorder that
occurs in 15-25% of patients [12] and typically occurs in the
setting of multisystem disease [15]. Tracheal involvement will
produce localized, segmental. or diffuse/circumferential areas of
tracheal narrowing (but the involvement is usually focal and
affects a 2-4cm segment of the rtrachea [15]). The subglottic
trachea is the most common site of involvement, but bronchial
stenosis has been reported in up to 18% of patients [12]. Wall
thickening is usually circumferential and can be smooth or nodular
[15]. The posterior membrane of the trachea is involved which aids
in distinguishing the condition from relapsing polychrondritis
[15]. Other authors suggest that bronchial wall thickening
involving segmental and subsegmental bronchi can be seen in 70% of
patients [17].
Pleural effusions are the most common pleural abnormality and are
present in 12-20% of patients [15].
Paranasal sinus involvement is characterized by mucosal thickening and variable opacification, but it can progress to destruction of cartilage and bone.
REFERENCES:
(1) J Thorac Imag 1995, 10: p.126-128
(2) AJR 1995 Feb;164(2):295-300
(3) Am Rev Respir Dis 1991; 143: 401-407
(6) ACR Syllabus #40: p.191
(7) Radiographics 1998; Frazier AA, et al. Pulmonary angiitis and granulomatosis: Radiologic-pathologic correlation. 18: 687-710
(8) Radiology 1999; Murphy JM, et al. Wegener granulomatosis: MR imaging findings in brain and meninges. 213: 794-799
(9) AJR 2000; Webb EM, et al. Using CT to diagnose nonneoplastic tracheal abnormalities: Appearance of the tracheal wall. 174: 1315-1321 (No abstract available)
(10) AJR 2001; Carazo ER, et al. Multiple renal masses as initial manifestation of Wegener's granulomatosis. 176: 116-118 (No abstract available)
(11) AJR 2001; Marom EM, et al. Diffuse abnormalities of the trachea and main bronchi. 176: 713-717 (No abstract available)
(12) AJR 2009; Ananthakrishnan L, et al. Wegener's granulomatosis in the chest: high-resolution CT findings. 192: 676-682
(13) Radiographics 2010; Castaner E, et al. When to suspect pulmonary vasculitis: radiologic and clinical clues. 30: 33-53
(14) Radiology 2010; Chung MO, et al. Imaging of pulmonary
vasculitis. 255: 322-341
(15) Radiographics 2012; Martinez F, et al. Common and uncommon
manifestations of Wegener granulomatosis at chest CT:
radiologic-pathologic correlation. 32: 51-69
(16) AJR 2013; Nemec SF, et al. Noninfectious inflammatory lung
disease: imaging considerations and clues to differential
diagnosis. 201: 278-294
(17) Radiographics 2020; Naeem M, et al. Noninfectious granulomatous diseases of the chest. 40: 1003-1019