Thallium Tumor Imaging:
Thallium Tumor Imaging General Considerations:
Thallium accumulates mainly within viable tumor tissue, less within connective tissue which contains inflammatory cells, and its accumulation is barely detectable in necrotic tissue. Cellular uptake of thallium is not affected by steroids, chemotherapy, or radiation therapy. Localization of Thallium within tumors is is likely multifactoral and in part related to blood flow, tumor viability, the sodium-potassium adenosinetriphosphatase system, the non-energy dependent co-transport system, the calcium ion channel system, vascular immaturity with leakage, and increased cell membrane permeability [1]. Radiation therapy and chemotherapy do not appear to immediately inhibit thallium uptake as they do gallium accumulation. A baseline pretreatment determination of a tumors thallium avidity is crucial to its efficacy in therapeutic response assessment. Early reports confirm an association between thallium uptake and histologic tumor response to treatment. The optimal time for thallium tumor imaging is 20 to 60 minutes post injection. Delayed images at 3 hours are recommended when imaging lymphoma because of an improved lesion to background ratio on the later images. The typical dose used for imaging is 3 to 4 mCi (or 30 to 40 uCi/kg). Spot views of the lesion should be 5 minute preset timed images using a high resolution collimator. The normal distribution of thallium within the body is choroid plexus of the lateral ventricles, lacrimal glands, salivary glands, thyroid, myocardium, liver, spleen, splanchnic areas, kidneys, and testes. There is also uniform muscle uptake. Bone marrow activity should not be seen, and if noted indicates marrow hyperplasia. There is little uptake in healing surgical wounds. The elimination of thallium is slow, with a biologic half-life of 10 days. The kidneys are the critical organ. Unfortunately, thallium does not demonstrate a 100% specificity for tumors and false-positive uptake has been seen in histiocytosis X, benign bone tumors, stress fractures, and inflammation.
REFERENCES:
1. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201 chloride and Tc-99m-sestamibi in tumor imaging. 181-234.
2. Semin Nucl Med 1993; Nadel HR. Thallium-201 for oncologic imaging in children. 23: 243-254
Breast Cancer:
Mammography has been accepted as the primary screening tool for breast cancer. Unfortunately, it is very difficult to mammographically distinguish benign from malignant lesions and the exam has a positive predicitve value for cancer of only 15 to 30%. Additionally, evaluation of patients with a dense fibroglandular pattern can also be difficult.
Studies have demonstrated the usefulness of thallium in differentiating benign from malignant breast lesions. Benign lesions seldom demonstrate tracer accumulation, although highly cellular adenomas and papillomas may demonstrate uptake of tracer. Overall Thallium has a sensitivity between 67 to 96% in the differentiation of benign from malignant breast lesions, and a specificity of 91-93%. Thallium is not useful for the detection of axillary node mets (sensitivity 50 to 60%). Lesion size may have a major effect on the exams sensitivity. In one study, the Thallium exam had a sensitivity of 67% for lesions greater than 1.5 cm in size, but only 20% for lesions below this size.
REFERENCES:
1. J Nucl Med 1993; Waxman AD, et al. Thallium scintigraphy in the evaluation of mass abnormalities of the breast. 34:18-23
2. J Nucl Med 1993; Lee VW, et al. A complementary role for thallium-201 scintigraphy with mammography in the diagnosis of breast cancer. 34: 2095-2100
3. J Nucl Med 1995; Maurer AH, et al. Limitations of craniocaudal thallium-201 and technetium-99m-sestamibi mammoscinitgraphy. 36: 1696-700
CNS Neoplasms:
Primary CNS Neoplasms:
The exam is performed approximately 20 minutes following the intravenous administration of 3 to 4 mCi of thallium. Rapid washout of tracer from CNS neoplasms has been described, and lesions may be missed if only delayed images are performed.
Normal brain uptake of the agent occurs from the CSF and is related to neuronal activity. There is normally little or no thallium uptake in the white matter. Thallium will not cross an intact BBB, yet disruption of the blood brain barrier is not the sole factor which affects thallium accumulation within a primary CNS lesion as little thallium accumulation is identified at sites of cerebral infarction. Uptake of thallium in CNS tumors is also likely dependent on the ATP-ase activity of the sodium-potassium pump and to active transmembrane transport via the K+/glucose co-transport system in viable tumor cells [1,2]. Thallium accumulates in residual recurrent tumor in porportion to the malignant grade and total viable tumor bulk. Thallium can also accumulate in benign tumors such as meningiomas and pituitary adenomas [3]. Thallium accumulation is minimal or negative at sites of radiation necrosis and resolving hematomas. Steroid administration does not affect thallium uptake. False positive exams have been described in association with inflammatory demyelinating disease [6].
Detectablity of brain lesions depends greatly upon lesion size and location. Lesions less than 2 cm in size, centrally located, or adjacent to areas of normally high activity (such as the base of the skull or vascular structures) may be missed. Malignant gliomas and meningiomas are detected with high sensitivity, while pituitary and parasellar tumors, low grade gliomas, and brainstem tumors are detected with low sensitivity. Intermediate results are seen with posterior fossa tumors and acoustic neuromas. Infratentorial lesions (such as medulloblastoma) are not well assessed- this may be related to the low grade nature of these lesions. Thus, low thallium uptake in these lesions does not necessarily confirm a non-malignant process. Thallium accumulation has been shown to be largely dependent on tumor grade, with low or no uptake in low grade tumors, and intense uptake in high grade lesions. Correlation with the patients CT/MRI is necessary
Quantitative assement of lesion activity has shown that most tumors will demonstrate a tumor-to-normal brain ratio of greater than 2.5, and ratios less than 1.5 suggest a non-malignant lesion [4]. Ratios are helpful for evaluating newly diagnosed, untreated lesions, but are not reliable for tumor grade when evaluating treated tumors. A higher ratio following therapy, however, is an absolute indication of tumor recurrence [5].
|
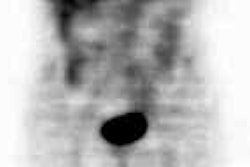
CNS malignancy: MR and thallium scan images from a patient with an enlarging brain lesion. On the MR image, note how edema makes detection of the primary tumor difficult. The thallium scan (right) demonstrates a focal area of tracer accumulation highly suspicious for CNS malignancy. A sterotactic biopsy was performed and the lesion was found to be a CNS lymphoma. |
|
|
REFERENCES:
1. J Nucl Med 1994; Tjuvajev JG, et al. Imaging of brain tumor proliferative activity with iodine-131-iododeoxyuridine. 35: 1407-17
2. Nucl Med Annual 1994; Mountz JM, et al. Brain SPECT: 1994 Update. 1-54 (p.43)
3. J Nucl Med 1995; Ishibashi M, et al. Thallium-201 in brain tumors: Rekationship between tumor cell activity in astrocytic tumor and proliferating cell nuclear antigen. 36: 2201-06
4. Semin Nucl Med 1993; Nadel HR. Thallium-201 for oncologic imaging in children. 23: 243-54
5. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201 chloride and Tc-99m-sestamibi in tumor imaging. 181-234 (p.198)
6. J Nucl Med 2003; Hayashi T, et al. Inflammatory demyelinating disease mimicking malignant glioma. 44: 565-569
CNS Lymphoma:
The risk of primary CNS lymphoma in HIV patients is 250- to 500
fold that of the average population in the United States [4].
However, HIV patients can develop both lymphoma and CNS infection-
such as toxoplasmosis [4]. Patients with toxoplasmosis infection
most commonly present with headache, fever, seizure, focal
neurologic defects, cranial nerve palsies, visual disturbances,
confusion, and psychomotor or behavioral changes [4].
Unfortunately, the two entities can have similar appearances on
imaging studies [4]. CNS lymphoma can have many of the same
manifestations [4]. Laboratory markers such as the presence of
Ebstein-Barr virus DNA in the CSF and a negative serologic result
for toxoplasmosis are helpful in confirming the diagnosis of CNS
lymphoma [4].
On CT or MRI, cerebral toxoplasmosis often produces multiple
nodular or ring-enhancing lesions associated with vasogenic edema
that is often disproportionate to the lesion size and resultant
mass effect [4]. The lesions are most commonly located in the
basal ganglia and frontal and parietal lobes [4]. CNS lymphoma can
have a similar appearance, but the lesions are most commonly
located in a multifocal or periventricular distribution [4].
Conventional DWI and apparent diffusion coefficient maps and
values have mixed results for differentiating primary CNS lymphoma
from toxoplasmosis [4]. On T2 weighted MR images, a pattern of
concentric zones of hypointensity and hyperintensity- called the
"concentric target" sign has been described in association with
toxoplasmosis infection, but needs further validation [4]. MR
spectroscopy will typically demonstrate decreased levels of
choline (a marker of cellular turnover) in toxoplasmosis lesions,
whereas CNS lymphoma generally have elevated choline levels
(although overlap can occur and spectroscopy is not completely
reliable) [4].
Thallium imaging can be used to aid in discriminating CNS
lymphoma (30%
incidence) from toxoplasmosis (60% incidence). Lymphoma will
avidly accumulate thallium,
while toxoplasmosis infection will typically demonstrate only mild
thallium uptake. The
lesion to contralateral non-lesion uptake ratio is generally
greater than 2.5:1 in cases of CNS lymphoma
[1] (other authors suggest a ratio of 2.9 or greater is 71%
accurate in suggesting the presence of lymphoma [4]).
Sequential thallium-gallium scanning may help to improve the
exams sensitivity and
specificity. Lymphomas will generally be thallium and gallium
positive, while
toxoplasmosis infection is thallium negative, but gallium positive
[3]. There have been
case reports of thallium accumulation in cerebral infections
including CMV encephalitis
(with a semiquantitative uptake ratio suggesting a malignant
lesion), candidiasis,
bacterial abscess, and toxoplasmosis [2]. Thallium imaging should
be delayed (3-4 hours
after injection) as early accumulation within inflammatory lesions
washes out, while
activity remains within neoplasms [3].
FDG PET imaging has also been used to discriminate between CNS
lymphoma and toxoplasmosis [4]. CNS lymphoma typically
demonstrates increased uptake greater than the normal cortex
(SUVmax 12.4-29.9), while toxoplasmosis demonstrates decreased
uptake compared to normal cortex (SUVmax 1.9-5.8) [4]. A lesion to
contralateral brain uptake ratio of less than 1.0 (0.3-0.7)
suggests toxoplasmosis infection, whereas lymphoma has uptake
ratios between 1.7-3.1 [4]. On delayed PET imaging can also be
used and will typically demonstrate progressive increased activity
over time, compared to toxoplasmosis infection [4].
REFERENCES:
1. AJR 1994; Aug.: p.417
2. J Nucl Med 1997; Gorniak RJ, et al. Thallium-201 uptake in cytomegalovirus encephalitis. 38: 1386-1388
3. Radiology 1999; Lee, VW, et al. Intracranial mass lesions:
Sequential thallium and
gallium scintigraphy in patients with AIDS. 211: 507-512
4. AJR 2021; Marcus C, et al. Imaging in differentiating cerebral
toxoplasmosis and primary CNS lymphoma with special focus on FDG
PET/CT. 216: 157-164
CNS Tumor Recurrence:
Thallium can be used to differentiate residual tumor from post-op/post-radiation therapy changes for CNS neoplasms such as gliomas. Radiation necrosis occurs 3 to 12 months following XRT and can be associated with exuberant gliosis. On CT scan or MRI, contrast enhancement can be seen in both radiation necrosis and recurrent tumor due to disruption of the blood brain barrier. Thallium uptake which is increased in comparison to the contralateral normal brain is highly suggestive of recurrence. Necrosis and inflammatory-infectious processes may rarely show increased uptake of thallium [1]. The sensitivity has been reported to be 69%, and the specificity 40% [5]
Combined Thallium-Tc-HMPAO imaging has also been evaluated in a small number of patients to confirm or exclude recurrent tumor. Thallium uptake was evaluated using the contralateral scalp activity for comparison, while Tc-HMPAO uptake was determined compared to cerebellar activity. A high likelihood of tumor recurrence was associated with a thallium to scalp ratio greater than 3.5:1. For patients with thallium uptake ratios between 1.1 to 3.4, a lesion to cerebellar Tc-HMPAO ratio less than 0.5 was indicative of no tumor recurrence (although one patient in the study in this group did have recurrent tumor). For thallium ratios between 1.1 to 3.4 and Tc-HMPAO ratios greater than 0.51, the study was not accurate in predicting tumor recurrence [2,3,4].
REFERENCES:
1. J Nucl Med 1995; Buchpiguel CA, et al. PET versus SPECT in
distinguishing radiation
encrosis from tumor recurrence in the brain. 36: 159-64
2. J Neurosurg 1992; Carvalho PA, et al. Detection of recurrent gliomas with quantitative thallium-201/technetium-99m HMPAO single-photon emission computerized tomography. 77: 565-570
3. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201 chloride and Tc-99m-sestamibi in tumor imaging. 181-234 (p.190)
4. AJNR 1991; Nov/Dec: p.1187-1192
5. AJR 1994; Kahn D, et al. Diagnosis of recurrent brain tumor: value ot 201Tl SPECT vs 18F-fluorodeoxyglucose PET. 163: 1459-1465
Cerebral Infarction:
In general, cerebral infarctions demonstrate little or no thallium uptake when the exam is performed within 5 days of the event, however, delayed studies performed 2 to 3 weeks post-event may demonstrate some thallium accumulation.
Infection/Inflammation:
Generally, inflammatory lesions have not been found to accumulate thallium. There have been case reports of thallium accumulation within CNS abscesses, inflammatory demyelinating diseases, and pulmonary actinomycosis. Tuberculosis may occasionally accumulate thallium [1]. Thallium uptake within both hilar and mediastinal adenopathy with sarcoid has also been described [2].
REFERENCES:
1. Radiol Clin North Am 1993; July: p. 865
2. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201 chloride and Tc-99m-sestamibi in tumor imaging. 181-234 (p.218)
3. J Nucl Med 2003; Hayashi T, et al. Inflammatory demyelinating disease mimicking malignant glioma. 44: 565-569
Kaposi's Sarcoma:
Kaposi's is a lymphoproliferative disorder associated with proliferation of spindle cells and vascular spaces. The incidence of Kaposi's has decreased in HIV patients (found in about 15% of HIV patients), but the overwhelming majority of cases (95%) still occur in homosexual men. Pulmonary involvement in seen in about 5% of cases and the disease is usually widespread by the time pulmonary involvement occurs. About 85% of patients with thoracic KS have cutaneous lesions and very low CD4 counts (below 100).
Thallium has been used to differentiate Kaposi's sarcoma from malignant lymphoma in AIDS patients. Kaposi's sarcoma is usually thallium positive and gallium negative in patients with no superimposed opportunistic pulmonary infection (Sensitivity about 90%). Lymphoma is generally positive on both examinations; but this may also be seen with TB or MAI infections, and in Kaposi's patients with a superimposed opportunistic pulmonary infection. Infection is usually gallium avid, but thallium negative. Thallium uptake may uncommonly be seen in association with certain pulmonary infections, however, the uptake is usually transient and delayed images will often demonstrate clearance of activity over time. Such clearance is typically not seen in association with thallium uptake in malignant lesions. This is most likely due to the different mechanisms of thallium uptake in neoplasms and inflammatory lesions. In inflammation, thallium accumulation is most likely related to passive diffusion into the extravascular space- with time, the tracer will diffuse back to the intravascular component and be eliminated [1].
False negative thallium scans for Kaposi's have been described with bronchial Kaposi's. Parenchymal Kaposi's is detected much better and the use of SPECT imaging can enhance lesion detection [2].
REFERENCES:
1. J Nucl Med 1996; Abdel-Dayem HM, et al. Evaluation of
sequential thallium and gallium
scans of the chest in AIDS patients. 37:1662-1667
2. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201 chloride and Tc-99m-sestamibi in tumor imaging. 181-234 (p.224)
Lung Carcinoma:
Thallium 201 imaging has a reported sensitivity between 91-100% for the detection of lung cancers. The exam is probably best reserved for lesions larger than 2 cm in size [2]. Unfortunately, a significant number of benign pulmonary conditions also demonstrate thallium uptake including TB, pneumonia (organizing pneumonia), silicosis, radiation pneumonitis, sarcoid, and granulomas [1,2]. Delayed imaging can aid in differentiation of infection from tumor as most malignant lesions will retain the thallium, while it will washout of inflammatory lesions [1]. Thallium imaging can be positive in about 50% of cases of bronchoalveolar cell carcinoma, which are characteristically not metabolically active on FDG imaging [2]. This may be because thallium uptake is not directly related to cell glycolysis [2]. Another drawback of thallium imaging is its poor sensitivity for the detection of nodal metastases [3].
REFERENCES:
1. J Nucl Med 1995; Chin BB, et al. Thallium-201 uptake in lung
cancer. 36: 1514-19 (No
abstract available)
2. J Nucl Med 2001; Higashi K, et al. Comparison of [18F]FDG PET and 201Tl SPECT in evaluation of pulmonary nodules. 42: 1489-1496
3. J Nucl Med 2001; Kubota K. Changing pattern of lung cancer and its imaging: 201Tl SPECT versus [18F]FDG PET. 42: 1497-1498 (No abstract available)
Lymphoma:
Overall, gallium imaging is superior to thallium in the evaluation of lymphomas. Thallium, however, does have a high affinity for low-grade non-Hodgkin's lymphomas (which typically have low gallium affinity). Thallium uptake in high or intermediate grade lymphomas is more variable, but these lesions typically demonstrate increased gallium accumulation. Thallium may also be beneficial in evaluating the mediastinum for residual disease. Normally, no thallium uptake should be seen in the mediastinum which appears photon deficient against the normal faint pulmonary activity. Gastrointestinal excretion of thallium limits it's usefulness for evaluation of abdominal lesions [1].
Thallium may also be beneficial in differentiating thymic rebound from recurrent disease in children postchemotherapy. The normal thymus is usually gallium avid, but will be negative on the thallium scan.
REFERENCES:
1. J Nucl Med 1996; Waxman AD, et al. Comparison of
gallium-67-citrate and thallium-201
scintigraphy in peripheral and intrathoracic lymphoma. 37: 46-50
Primary Malignant Bone and Soft Tissue Tumors:
Thallium is more accurate than Tc-MDP and gallium in determining extent of involvement of primary bone tumors and in following respose to chemotherapy as it does not demonstrate activity secondary to bone healing [1]. On initial imaging of osteosarcoma, the presence of central tumor photopenia can be seen in up to 52% of cases [4]. Central tumor photopenia is suggestive of central tumor necrosis and may be negatively associated with patient survival [4]. In osteosarcoma, thallium uptake usually decreases significantly in tumors which have shown a histologic response to chemotherapy. Research indicates that patients with more than 90% necrosis following preoperative chemotherapy have a better prognosis and Thallium imaging can be used to assess the degree of necrosis [2]. SPECT images can be performed to permit co-registration with CT or MRI images. Unfortunately, thallium accumulation in bone lesions is non-specific and has been described in some benign lesions including fractures. Marked thallium uptake has been described in Paget's disease, fibrous dysplasia, and acute osteomyelitis. Additionally, the high radiation exposure from the exam has produced concern for risk of subsequent cancer development [5].
Thallium has also been used in the evaluation of soft tissue sarcomas. Uptake within the lesion appears to reach a maximum by about 1 hour after injection. Lesion to muscle ratios greater than 3:1 are typically identified with sarcomas, while infection or inflammation have ratios below 1.0. Uptake in pulmonary metastases is poor. Serial thallium examinations during therapy are useful for assessing tumor response to treatment (ie: tumors that are responding will show decreased activity) [3].
REFERENCES:
1. Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201
chloride and
Tc-99m-sestamibi in tumor imaging. 181-234 (p.203)
2. J Nucl Med 1996; Ohtomo K, et al. Thallium-201 scintigraphy to assess effect of chemotherapy in osteosarcoma. 37: 1444-48
3. J Nucl Med 1998; Sumiya H, et al. Midcourse Thallium-201 scinitgraphy to predict tumor response in bone and soft tissue tumors. 39: 1600-1604
4. AJR 2007; McCarville MB, et al. The cause and clinical significance of central tumor photpenia on thallium scintigraphy of pediatric osteosarcoma of the extremity. 188: 572-579
5. AJR 2010; Kaste SC, et al. Estimation of potential excess cancer incidence in pediatric 201Tl imaging. 194: 245-249
Thyroid Carcinoma:
The advantages of Thallium scintigraphy for imaging thyroid cancer patients include low radiation exposure compared to I-131 and patients do not need to be removed from thyroid hormone replacement therapy. However, in vitro studies suggest that thallium uptake by thyroid cells is increased by TSH and that withdrawl of thyroid hormone has the potential to improve the sensitivity of 201Tl scintigraphy for detecting thyroid remnant or cancer [5]. Additionally, about 25% of metastases and recurrences from well differentiated thyroid carcinoma may not show iodine uptake [1]. Primary thyroid carcinoma mets have been detected using thallium with a sensitivity between 35 to 95%, with specificities between 94-97%. For selected anatomic areas, thallium scanning is probably at least as sensitive as I-131 in the detection of thyroid carcinoma and thallium imaging has been shown to be roughly equivalent to FDG-PET for lesion detection (although PET images have higher spatial resolution and better contrast) [4]. Thallium is not recommended as the only modality for the follow-up of patients with thyroid cancer, but is probably best used to identify the presence of cervico-mediastinal lymph node mets [2].
Unfortunately, thallium uptake is not specific for thyroid cancer and does not give predictive information on the therapeutic potential of I-131. Additionally, it is less sensitive than I-131 scanning in the detection of residual thyroid tissue, diffuse pulmonary metastases, bone metastases, liver mets, and for metastases below the hemidiaphragms (due to the high background activity in the abdomen and pelvis). It is probably best to limit thallium scans to the head, neck, and chest in order to obtain higher count density images of these regions [2]. In patients with Hurthle cell carcinoma of the thyroid, thallium scanning should be performed as iodine scanning is invariably negative. Thallium scanning may also be beneficial in patients with elevated thyroglobulin levels, but negative I-131 scans [2]. (See also Tc-99m-Tetrofosmin imaging for thyroid cancer)
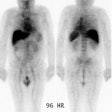
|
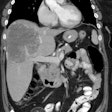
Recurrent thyroid cancer: The patient below had a history of thyroid cancer with surgical resection and prior I-131 ablation. Because he relapsed with massive disease (a large neck mass can be seen on the patients CT scan), there was reluctance to remove the patient from thyroid hormone replacement therapy. A Tl-201 exam diagnostic exam (left) was performed and revealed massive recurrent disease within the lower neck and mediastinum. |
|
|
REFERENCES:
(1) Nucl Med Annual 1994; Abdel-Dayem HM, et al. Role of Tl-201
chloride and
Tc-99m-sestamibi in tumor imaging. 181-234 (p.212)
(2) J Nucl Med 1988: Brendel AJ, et al. Thallium-201 imaging in the follow-up of differentiated thyroid carcinoma. 29: 1515-20
(3) J Nucl Med 1998; Unal S, et al. Thallium-201, Technetium-99m-tetrofosmin and Iodine-131 in detecting differentiated thyroid carcinoma metastases. 39: 1897-1902
(4) J Nucl Med 2001; Shiga T, et al. Comparison of 18F-FDG, 131I-Na, and 201-Tl in diagnosis of recurrent or metastatic thyroid cancer. 42: 414-419
(5) J Nucl Med 2002; Mruck S, et al. Uptake of 201Tl into primary cell cultures from human thyroid tissue in multiplied by TSH. 43: 145-152