PET Imaging in Other Neoplasms:
Bladder cancer:
Transitional cell carcinoma accounts for more than 90% of bladder cancers [1]. The tumors are commonly multifocal and there is a high local recurrence rate [1]. High grade tumors are those that invade the deep layers of the bladder wall and these lesions have a high potential for metastatic spread [1]. At least 50% of high grade tumors may have occult metastatic disease at the time of initial diagnosis [1]. Evaluation of bladder neoplasms is difficult due to urinary excretion of FDG which can obscure the tumor [1]. Two hour post injection imaging following oral hydration with 800-1000 mL of water and lasix administration (20 mg) has been shown to markedly reduce the concentration of FDG in the urine and allow visualization of bladder lesions and local lymph node metastases [1]. However, PET/CT findings can affect patient staging and treatment planning [2]. PET/CT findings can change stage in up to 15% of patients and alter management in up to 17% of patients [2].
REFERENCES:
(1) J Nucl Med 2007; Anjos DA, et al. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. 48: 764-770
(2) AJR 2009; Vikram R, et al. Imaging and staging of transitional cell carcinoma: part I, lower urinary track. 192: 1481-1487
Cholangiocarcinoma:
For detecting cholangiocarcinoma FDG PET has a sensitivity of
61% and a specificity of 80% [2]. PET scans are generally
positive in patients with nodular lesions (sensitivity 85%), but
can be false negative in cases of infiltrating tumor
(sensitivity 18%) [1,2]. False positive findings are associated
with biliary stents and with acute cholangitis [1]. PET findings
can result in a change in patient management in up to 30% of
cases [1].
Higher tumor FDG uptake is associated with a worse prognosis
[4].
Greater reduction in SUV following treatment initiation is
associated with better overall survival [4]. For cancers treated
with GEMOX-B, the reduction in SUVmax after therapy has been
shown to be a better predictor for survival than morphologic and
density changes [3].
|
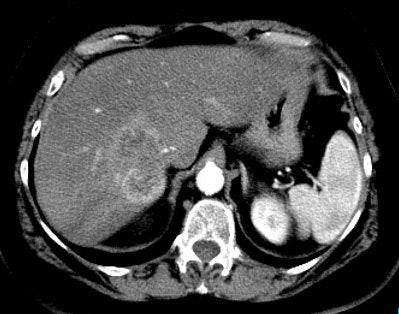
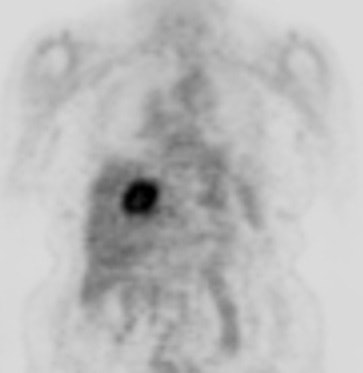
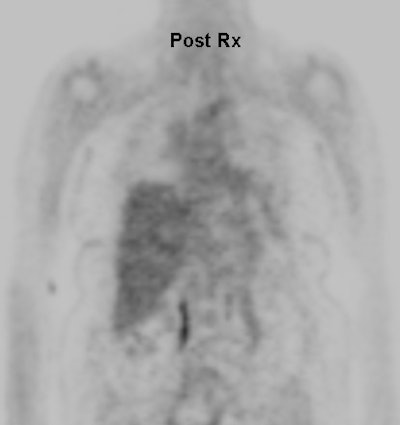
Cholangiocarcinoma: The patient below had a large cholangiocarcinoma. FDG PET imaging demonstrated markedly increased tracer uptake by the mass. There was no evidence of metastatic disease on PET imaging and the patient demonstrated a dramatic response to chemotherapy. |
|
|
REFERENCES:
(1) J Gastrointest Surg.2004; Anderson CD, et al. Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. 8: 90-97
(2) Radiol Clin N Am 2004; Hustinx R. PET imaging in assessing
gastrointestinal tumors. 42: 1123-1139
(3) AJR 2015; Sahani DV, et al. Measuring treatment response to
systemic therapy and predicting outcome in biliary tract cancer:
comparing tumor size, volume, density, and metabolism. 204:
776-781
(4) J Nucl Med 2017; Jo J, et al. Prospective evaluation of the
clinical implications of the tumor metabolism and
chemotherapy-related changes in advanced biliary tract cancer.
58: 1255-1261
Endometrial cancer:
Endometrial cancer is the most common female pelvic malignancy
and the fourth most common cancer in women [1,6]. The most
common presenting symptom is abnormal bleeding (80% of cases)
[1]. About 15% of post-menopausal women presenting with abnormal
bleeding have endometrial carcinoma [1]. Hysterectomy and
bilateral salpingo-ooporectomy is the treatment for patients
with presumed stages I-III [6]. In general, early-stage
endometrial cancer has a good prognosis with a 5-year survival
of more than 90% for patients with FIGO stage Ia or Ib [5].
However, survival decreases to 57% with pelvic LN metastases and
49.4% with abdominal LN mets [9].
Prognostic factors include histologic type, depth of
endo/myometrial invasion, extension into the cervix, involvement
of pelvic/paraaortic lymph nodes, and distant metastases
[1,8,9]. Pelvic lymph nodes are the most common site for
extrauterine disease at presentation [8]. The survival rates of
patients with lymph node metastases is significantly lower than
in patients without nodal mets (the 5 year survival rate drops
to about 50% for this patient subgroup [8]) [2].
Lymph node metastases: Patients with high risk disease include
those with grade 3 endometroid, serous papillary, clear cell, or
carcinosarcoma endometrial cancer [9]. The depth of myometrial
invasion is an important factor in predicting lymph node
metastases- increasing from 3% with superficial myometrial
invasion (stage IB), to more than 40% with deep myometrial
invasion (stage IC) [2]. Because the lymphatic drainage of the
uterus is complex, paracaval and paraaortic lymph nodes can be
involved without involvement of pelvic LNs [9].
Conventional imaging has sensitivities between 18-66% for
detection of lymph node mets (specificity 73-99%) [2]. Because
surgical lymphadenectomy has not been shown to be associated
with a survival benefit [8] and can incur perioperative
complications and long-term morbidity such as lymphedema, some
centers will perform the procedure when the primary tumor
demonstrates high risk features such as high grade histology
(between 3-5% of patients with high grade histology harbor, a
tumor larger than 2 cm, deep (>50% thickness) myometrial
invasion, or cervical stromal spread [6]. Between 20-25% of
patients will develop recurrence following primary treatment,
usually within the first 3 years [6]. The majority of
recurrences are in patients with higher risk tumors (there is
only a 5% risk in patients with stage I tumors) [6]. The most
common site for recurrence is the lymph nodes and the vagina
[6].
FDG does localize to endometrial tumors and is useful for postsurgical monitoring and surveillance for recurrent disease [1]. In one study, PET confirmed recurrence in 88% of cases, helped localize the site of disease, and detected asymptomatic recurrences in 12% [1]. Additionally, additional metastatic sites were found in 35% of patients which resulted in a significant change in management [1].
In the detection of lymph node metastases in newly diagnosed
patients with endometrial cancer, PET/CT has a reported
sensitivity of 53-85%, specificity of 91-99.6%, and an accuracy
of about 89-98% [2,7,9] (in one study the patient based
sensitivity was 50%, specificity 87%, and accuracy 77.5% [2]). A
meta-analysis found an overall pooled sensitivity of 72% and
specificity of 94% [8]. PET/CT has been shown to be superior to
CT for detection of LN metastases [9]. Metastatic lesion size
has been shown to affect lesion detectability. Sensitivity for
metastases 4 mm or smaller has been reported to be only 13-17%,
but increased to 93-100% for lesions 10mm or larger [2,8]. The
SUVmax, MTV (over 30 mL), and TLG are significantly related to
the presence of deep myometrial invasion, lymph node metastases,
and high histologic grade [7,8].
In uterine cancer, a decrease in SUV after neoadjuvant
chemotherapy before surgery was shown to correlate better than
MRI with histologic response [4]. Patients without residual
abnormalities on FDG PET had an estimated 80% 5 year survival
compared to 32% in patients with residual abnormalities [4].
For the detection of recurrent endometrial cancer, a
meta-analysis found a pooled sensitivity of 95% and a
specificity of 91% [8].
18F-estradiol (FES) has also been studied for the evaluation of endometrial cancer [3]. ER expression and glucose metabolism are generally opposite- i.e. - low glucose metabolism and high ER expression in benign tumors [5]. Most endometrial carcinomas demonstrate significantly greater FDG uptake compared to FES [3]. However, significantly higher FES uptake and low FDG uptake is seen in endometrial hyperplasia [3]. These findings are consistent with reduced estrogen dependency as the tumor progresses to a higher stage or grade [5].
REFERENCES:
(1) J Nucl Med 2005; Pandit-Taskar N. Oncologic imaging in gynecologic malignancies. 46: 1842-1850
(2) AJR 2008; Kitajima K, et al. Accuracy of 18F-FDG PET/CT in detecting pelvic and paraaortic lymph node metasases in patients with endometrial cancer. 190: 1652-1658
(3) Radiology 2008; Tsujikawa T, et al. Uterine tumors: pathophysiologic imaging with 16 α - [18F] fluoro-17 β - estradiol and 18F fluorodeoxyglucose PET- initial experience. 248: 599-605
(4) J Nucl Med 2009; Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. 50: 88-99
(5) J Nucl Med 2009; Tsujikawa T, et al. Functional images
reflect aggressiveness of endometrial carcinoma: estrogen
receptor expression combined with 18F-FDG PET. 50:
1598-1604
(6) J Nucl Med 2015; Lee SI, et al. Evaluation of gynecologic
cancer with MR imaging, 18F-FDG PET/CT, and PET/MR
imaging. 56: 436-443
(7) J Nucl Med 2015; Husby JA, et al. Metabolic tumor volume on
18F-FDG PET/CT improved preoperative identification
of high-risk endometrial carcinoma patients. 56: 1191-1198
(8) J Nucl Med 2016; Bollineni VR, et al. High diagnostic value
of 18F-FDG PET/CTin endometrial cancer: systematic
review and meta-analysis of the literature. 57: 879-885
(9) Radiology 2017; Atri M, et al. Utility of PET/CT to
evaluate retroperitoneal lymph node metastases in high-risk
endometrial cancer: results of ACRIN 6671/GOG 0233 trial. 283:
450-459
Gastric carcinoma:
In the Western world, gastric cancer has a poor prognosis with about 80% of patients presenting with advanced stage disease [3]. Gastric adenocarcinomas display extreme genetic complexity and biologic heterogeneity, and FDG avidity is dependent on the biologic and clinical-pathologic characteristics of the individual tumor [7]. Patients with signet ring cell tumors have a lower response rate to treatment and a worse prognosis [4].
T-stage:
Unfortunately, a relatively large number of gastric cancers are not avid for FDG (gastric cancer is not FDG avid in up to 53% of cases [5]) [4]. Published sensitivities for FDG PET range from 47-96% (mean 77%) [4]. PET results are in part related to the type of lesion- FDG PET imaging can detect about 50% of early gastric cancers and up to 98% of advanced gastric cancers (this is similar to CT) [2,5]. Uptake in signet ring cell or mucinous tumors tends to be low (due to lower glucose transporter-1 expression [7]) [1,2,3,4]. Other lesions with low FDG uptake are tumors with a high mucinous content [4]. The intestinal subtype of gastric cancer appears to be associated with higher FDG uptake [5]. Overall, PET imaging is not helpful in determination of the T-stage of the lesion and endoscopic US is presently the most reliable method for determining T-stage [3].
18F-FLT shows excellent uptake in gastric cancers (up to 100% sensitivity [4])- even in tumors with signet ring features [4]. Unfortunately, high uptake in the liver renders this agent of little use for the detection of hepatic metastatic disease and there is also high uptake in the bone marrow.
N-stage:
The number and location of lymph node metastases carries significant prognostic information [3]. For lymph node staging, FDG PET detection of N1 nodes (perigastric) is poor (sensitivity of about 35%) [2]. This is because these nodes lie in close proximity to the stomach and uptake within the primary tumor may obscure adjacent nodal upatke [3]. The identification of these nodes may not be crucial as they should be removed at the time of surgery [3]. The sensitivity for N2 disease is also about 35%, but it approaches 50% for N3 disease [2]. The identification of non-perigastric nodes can have significant impact on patient management and surgical planning [3].
Metastatic disease:
The most common location for gastric cancer metastases is the
liver [3]. Less common sites include the lung, adrenal glands,
and bones [3]. Peritoneal metastases can also be seen [3].
Recurrence:
There is decreased sensitivity for the detection of tumor
recurrence in tumors with initial low FDG uptake compared to
those with high FDG uptake [7].
Prognosis:
Gastric cancer recurs in 12-48% of patients following curative
surgical resection [6]. 18F-FDG uptake by gastric
cancer is related to tumor aggressiveness and histopathology
[6]. A high tumor to liver uptake ratio (greater than 2.0) has
been shown to predict risk for recurrence in patients with
advanced gastric cancer [6].
REFERENCES:
(1) J Nucl Med 2005; Yun M, et al. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET: 46: 953-957
(2) J Nucl Med 2005; Yun M, et al. Lymph node staging of gastric cancer using 18F-FDG PET: a comparison with CT. 46: 1582-1588
(3) Radiographics 2006; Lim JS, et al. CT and PET in stomach cancer: preoperative staging and monitoring of response to therapy. 26: 143-156
(4) J Nucl Med 2007; Herrmann K, et al. Imaging gastric cancer
with PET and the radiotracers 18F-FLT and
18F-FDG: a comparative analysis. 48: 1945-1950
(5) J Nucl Med 2015; Kaneko Y, et al. Improving patient
selection for 18F-FDG PET scanning in the staging of
gastric cancer. 56: 523-529
(6) J Nucl Med 2015; Lee JW, et al. Relationship between 18F-FDG
uptake on PET and recurrence patterns after curative resection
in patients with advanced gastric cancer. 56: 1494-1500
(7) J Nucl Med 2016; Kim SJ, et al. Primary tumor 18F-FDG
avidity affects the performance of 18F-FDG PET/CT
for detecting gastric cancer recurrence. 57: 544-550
Gastrointestinal Stromal Tumors
Gastrointestinal stromal tumors account for 0.1 to 3% of all
gastrointestinal tract tumors [2], but they are the most common
mesenchymal neoplasm of the gastrointestinal tract [1]. They
arise from the muscularis propria (outer muscular layer- likely
from the cells of Cajal [2]) and most commonly have an exophytic
growth pattern [1]. GISTs are defined by their expression of
C-KIT (CD117)- a tyrosine kinase growth factor receptor (100% of
tumors) [1,2,7] and CD34- a hematopoietic progenitor cell
antigen (70% of tumors) [7]. Patients with neurofibromatosis
type 1 have an increased prevalence of GISTs (the tumors are
typically multiple and tend to be located predominantly in the
small intestine) [1,5]. The most common location for GISTs is
the stomach (70%), followed by the small intestine (20-30%),
anorectum (7%), colon, and esophagus (less than 5%) [1,2].
Patients tend to be middle-aged or older [2].
Lesions under 5 cm are typically asymptomatic. Larger lesions
can be associated with bleeding, anemia, and dyspepsia [2].
GIST's can be benign (70% of cases) or malignant (30% of cases)
[2]. Malignant lesions tend to recur and metastasize- most
commonly to the liver and peritoneum [2].
The most common presentation is a heterogeneous exophytic mass arising from the gastric wall. Necrosis and hemorrhage results in the presence of cystic spaces [1]. Calcification is not common (3% of cases) [1].
In patients with recurrent GIST lesions, FDG PET imaging has a sensitivity of 86% and a specificity of 98% for the identification of sites of tumor involvement- this is similar to CT [2]. False negative PET scans can be seen in association with small lesions [2].
|
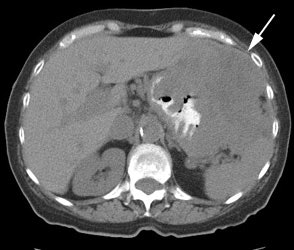
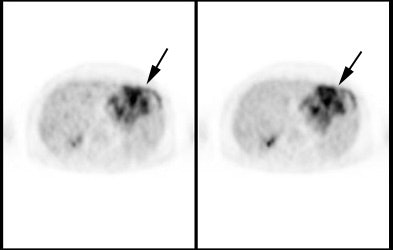
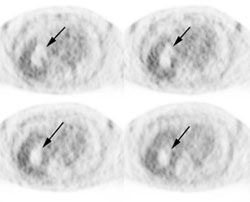
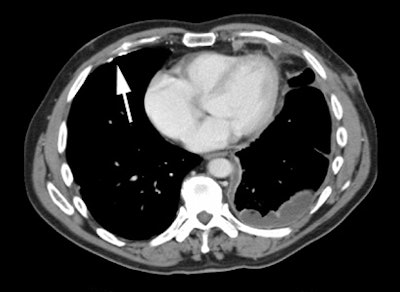
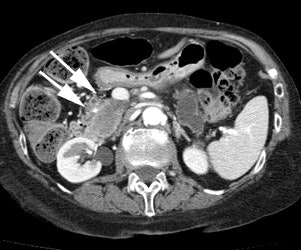
Gastrointestinal stromal tumor: The patient below had a large gastric GIST tumor (white arrow). FDG PET imaging demonstrated markedly increased, heterogeneous tracer uptake by the mass (black arrows). There was no evidence of metastatic disease. |
|
|
PET in treatment response:
Imatinib is a tyrosine kinase inhibitor that targets Bcr-ABL,
c-KIT, and platelet derived growth factor alpha (PDGFA) used in
the treatment of GIST tumors [11]. Depending on the type of
driver mutation, the partial response rate is up to 84% [11].
Following initiation of treatment, the early identification of
non-responders can permit modification of treatment to include
an increase in dosage [3] or discontinuing expensive ineffective
treatment [7].
FDG PET imaging predicts response to therapy earlier than CT in
up to 22.5% of patients [2] (this is because change in tumor
size is not a reliable indicator of tumor response [4]).
Following initiation of Gleevac therapy, the most dramatic
changes in responding lesions are seen within the individual
tumor masses that become homogeneous and hypodense on CT, while
the decrease in tumor size may be minimal [10]. In fact, volume
response measureable by CT often requires 6-9 months of Imatinib
treatment [11]. Therefore, the normal RECIST criteria do not
readily represent whether a GIST tumor is responding to therapy
(30% decrease in size) and the Choi response criteria have been
developed [10]. In the Choi criteria, a response is defined as a
10% decrease in unidimensional tumor size, or a 15% decrease in
CT attenuation [10]. Tumor progression is defined as the
appearance of new lesions or metastases, the appearance of new
intratumoral nodules or an increase in the size of an existing
intratumoral nodule, or an increase in overall tumor size by
more than 20% in the absence of post-treatment hypodense changes
[10].
PET imaging is a sensitive method to monitor response to therapy in patients with GIST tumors [3,4,9] and is superior to CT imaging and RECIST criteria in predicting early response [7,8,9,11]. Effective imatinib therapy results in a rapid decreased in tumor FDG uptake in responding lesions (within 1-8 days)- well before changes in tumor volume are observed [6,11]. Marked decreased tumor uptake can be seen within one week of initiation of imatinib mesylate (Gleevae) therapy [9]. Accurate tumor response can be predicted in 85% of patients after 1 month of therapy, and in up to 100% of patients when imaged between 3 and 6 months following initiation of treatment [3]. A good response to therapy demonstrated on PET imaging is associated with a longer progression-free survival (92% versus 12% after 1 year) [6,8]. The preoperative use of imatinib mesylate has also been shown to improve resectability and reduce surgical mortality [9]. Although PET imaging has been shown to be superior to CT imaging for the detection of tumor response, a dual-modality PET/CT exam is the most accurate method for assessing the effects of therapy [3].
REFERENCES:
(1) Radiographics 2003; Levy AD, et al. Gastrointestinal stromal tumors: radiologic features and pathologic correlation. 23: 283-304
(2) J Nucl Med 2004; Gayed I, et al. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. 45: 17-21
(3) J Nucl Med 2004; Antoch G, et al. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of Imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. 45: 357-365
(4) AJR 2004; Choi H, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. 183: 1619-1628
(5) AJR 2005; Levy AD, et al. Gastrointestinal stromal tumors in patients with Neurofibromatosis: imaging features with clinicopathologic correlation. 183: 1629-1636
(6) Radiol Clin N Am 2005; Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. 43: 189-204
(7) AJR 2006; Lassau N, et al. Gastrointestinal stromal tumors
treated with Imatinib: monitoring response with
contrast-enhanced sonography. 187: 1267-1273
(8) J Nucl Med 2011; Eary JF, Conrad EU. Imaging in sarcoma.
52: 1903-1913
(9) J Nucl Med 2012; Van den Abbeele AD, et al. ACRIN 6665/RTOG
0132 Phase II Trial of Neoadjuvant Imatinib Mesylate for
Operable Malignant Gastrointestinal Stromal Tumor: Monitoring
with 18F-FDG PET and Correlation with Genotype and GLUT4
Expression. 53: 567-574
(10) AJR 2012; Nishino M, et al. Personalized tumor response
assessment in the era of molecular medicine: cancer-specific and
therapy-specific response criteria to complement pitfalls of
RECIST. 198: 737-745
(11) J Nucl Med 2018; Farag S, et al. Early evaluation of
response using 18F-FDG PET influences management in
gastrointestinal stromal tumor patients treated with neoadjuvant
Imatinib. 59: 194-196
Hepatocellular carcinoma:
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and about 70% of the cases are associated with underlying chronic liver disease [5]. In the United States, HCC has exhibited the largest increase in incidence over the past 10 years, in part related to the epidemic of hepatitis B and C infections [13]. Screening US has limited sensitivity for the detection of HCC, especially for smaller lesions. The sensitivity of contrast enhanced CT is around 70%, and for contrast enhanced MR approximately 80% [11]. Unfortunately, an additional 30-50% of unsuspected intrahepatic sites of HCC (mostly smaller than 2 cm) are found at transplantation [11].
Metastases occur most commonly to the lung (55%), abdominal
lymph nodes (about 40%), and bone (about 30% of patients and
lesions are lytic) [15]. Bone mets are most commonly encountered
in the setting of multiple other non-osseous sites of metastatic
disease [15]. However, isolated bone metastases may be more
common than previously thought and can be found in up to 12% of
patients [14]. Distant nodal mets can be seen in about 10% of
patients- most commonly to mediastinal or cardiophrenic angle
nodes [15]. Adrenal mets are found in about 10% of cases [15].
Brain metastases are rare [15]. Most patients with metastatic
disease have advanced stage tumors (Stage III or IV) [15].
18F-FDG:
Varying accumulation of FDG has been described in
hepatocellular carcinomas (HCC) depending on the degree of tumor
differentiation (due to variable degrees of
glucose-6-phosphatase activity) [1,2]. Glucose-6-phosphatase
(which is found in high concentrations in normal liver cells)
metabolizes phosphorylated FDG and results in poor intracellular
accumulation of the agent [9]. Well-differentiated HCC's are
histologically very similar to normal liver cells and may
contain an abundance of glucose-6-phosphatase (which
dephosphorylates intracellular 18F-FDG-6-phosphate
that can then leak back into the circulation) [4,12]. Hence,
well differentiated HCC's can have uptake similar to normal
liver and may not be detected on FDG-PET exams [1,2,8]. Other
authors suggest that fructose-1,6-biohosphatase 1 in well
differentiated tumors may inhibit FDG uptake [22].
Treatment:
Because the majority of patients present with advanced stage disease treatment options are limited [5]. Even in patients that can undergo curative resection, up to 50% of patients develop intrahepatic recurrence from second primaries or from intrahepatic spread [5]. Certain patients with unresectable lesions may be candidates for liver transplantation [5]. The Milan criteria have been developed to identify patients that would most benefit from liver transplant as treatment fo HCC [17]. Patients with a single tumor diameter of 5 cm or less, or a maximum of three tumors each with a diameter of 3 cm or less have been shown to have a 5-year survival rate of 75% and a disease-free survival rate of 83% [17]. In patients being considered for liver transplant for hepatocellular carcinoma accurate assessment for the presence of distant metastases is vital [5]. Recent estimates of the costs for each year of life gained after liver transplantation for HCC range from $44,000 to $183,000 [5]. In this setting, the marginal costs associated with PET imaging become negligible [5].
Various palliative treatments exist for patients with inoperable HCC including transcatheter arterial chemoembolization (TACE), intraarterial chemotherapy with concurrent external-beam radiotherapy (CCRT- one study suggested that patients with high FDG uptake have a survival benefit from CCRT compared to TACE [20]), systemic chemotherapy, immunotherapy, and radiofrequency thermal ablation [12]. FDG has also been studied to monitor response to therapy.
TACE can be effective as either a palliative or curative
treatment for HCC, however, the 6 month recurrence rate has been
reported to be about 22% and the 12 month recurrence rate is
about 78% [12]. The ability of CT to determine tumor viability
after TACE is limited due to the retained hyperattenuating
lipiodol material that makes detection of enhancing viable tumor
difficult [12]. Absent FDG uptake is a sign of effective therapy
and greater than 90% tumor necrosis [12]. FDG uptake greater
than or equal to the surrounding liver is suspicious for
residual viable tumor [12]. FDG uptake on post therapy scans is
also an independent prognostic factor for decreased progression
free and overall survival [20].
However, following TACE, HCCs undergo ischemic necrosis and the surrounding liver tissue may become inflamed- especially during the early post-embolic period (within 3 months following the procedure) [12]. Hence- false positive exams may occur secondary to inflammatory changes associated with the procedure [12]. Ideally, PET imaging should be performed approximately 24 hours following the procedure to decrease uptake associated with post-Rx inflammation and to best identify residual tumor that could be immediately re-treated [16].
Additionally, the presence of high attenuation, material such
as lipiodol, may result in a PET/CT artifact that falsely
elevates apparent activity in the lesion [12]. Review of
non-attenuation corrected images is important in differentiating
artifact from residual tumor (unfortunately, non-attenuation
corrected images are less useful for central lesions) [12].
Respiratory motion is another potential artifact can affect
lesions in the liver dome [12]. Respiratory motion artifacts can
result in under-correction of attenuation of the upper liver,
because of larger lung volumes on the CT scan than on the PET
emission images [12].
Stereotactic ablative radiotherapy (SART) is a new radiation
therapy technique using the highly precise delivery of high-dose
ratiation therapy to treat unresectable HCC [19]. FDG imaging
prior to treatment may help to identify patients that may be at
increased risk for SART failure [19]. A tumor SUVmax of greater
than 3.2 is associated with a decreased tumor control rate
following standard SART [19]. Dose escalation may be required in
those cases in order to improve tumor kill [19].
Other agents for imaging HCC:
18F-fluorocholine:
18F-fluorocholine has been studied for the detection of HCC. Choline is one of the components of phosphatidylcholine- an essential element of phospholipids in the cell membrane [11]. Because of a higher choline content in HCC than in normal liver, agents labeled to choline may be superior to FDG for the detection of HCC [11]. In one study the overall patient based sensitivity for 18F-fluorocholine was 88%, compared to 68% for FDG [11]. For the detection of well differentiated HCC the site based sensitivity of 18F-fluorocholine was 94%, compared to 59% for FDG [11].
One drawback of the agent is that decreased tracer uptake can also be observed with malignant lesions- including hepatocellular carcinoma, cholangiocarcinoma, and metastatic disease [11]. Another drawback of the agent is that tracer uptake can also occur in benign lesions such as FNH (up to 88% of FNH lesions), hepatic adenoma, and inflammatory conditions such as cholangitis [11].
18F-fluorothymidine:
18F-fluorothymidine will show variable uptake in
hepatocellular carcinoma, however, normal liver uptake limits
the usefulness of the agent [10].
68Ga-PMSA:
68Ga-PMSA uptake can be seen in other tumors [23]. 68Ga-PMSA
has been shown to be superior to FDG for the detection of HCC
[23]. In one study, 97% of HCCs and no regenerative nodules
demonstrated 68Ga-PMSA uptake (only 27% of the
lesions were FDG positive) [23]. 68Ga-PMSA uptake
appears to correlate with areas of arterial phase hypervascular
enhancement, suggesting uptake in tumoral microvessels [23].
11C-agents:
11C-acetate:
11C-acetate has shown promise in the ability to detect well-differentiated HCC's [4]. CT imaging for hepatocellular carcinoma can be limited by a background of cirrhosis intermixed with regenerative and dysplastic nodules that can obscure small lesions [17]. 11C-acetate imaging is not affected by cirrhosis [17]. The de novo synthesis of free fatty acids is upregulated in well-differentiated HCC and acetate, a precursor of acetyl-coenzyme A, may be the preferred metabolic substrate [17] (11C-acetate is a metabolic substrate of beta-oxidation and a precursor of amino acid and sterols [5]). Biochemical pathways for acetate accumulation include: 1- esterification to form acetyl CoA as a major precursor in beta-oxidation for fatty acid synthesis (most dominant method for tumor uptake); 2- entering the Krebs cycle from acetyl coenzyme A (acetyl CoA) or as an intermediate metabolite; 3- combining with glycine in heme synthesis; and through citrate for cholesterol synthesis [4]. The reported sensitivity for 11C-acetate PET for the detection of hepatocellular carcinoma is about 75-87% [9,17]. Smaller lesions may not be detected- in one study, sensitivity for lesions 1-2 cm in size was only 32% [9], however, other authors report detection rates of 87% for small lesions (1-2cm) [17]. Overall, 11C-acetate imaging has been shown to be surperior to CT for the detection of small HCC [17].
11C-acetate PET is superior to FDG PET for the
detection of well-differentiated HCC [9,17]. In one study, 11C-acetate
imaging was able to detect all hepatocellular carcinomas that
were FDG negative (these proved to be well-differentiated
lesions) [4]. Interestingly, in that same study many lesions
demonstrated both FDG and 11C-acetate uptake
indicative of varying morphology within the lesion [4].
Combined FDG/11C-acetate PET imaging may yield the
highest sensitivity for detection of hepatocellular carcinoma- 11C-acetate
for the detection of the more well-differentiated lesions and
FDG for the detection of dedifferentiated tumors [9,17].
In a small number of patients, 11C-acetate imaging
appears to be negative in liver metastases, cholangiocarcinoma,
and hemagioma [4]. 11C-acetate uptake can be
seen in up to 94% of cases of FNH and can also occur in hepatic
adenomas [11] and in hemangioma [13]. Regenerating nodules do
not appear to be 11C-acetate avid [17]. Mild 11C-acetate
may be seen in high-grade dysplastic nodules [17].
For the evaluation of metastatic disease, 11C-acetate has been shown have excellent sensitivity for the detection of isolated bone metastases (93%, compared to 62% for FDG PET) [14]. However, the sensitivity decreases in patients with multiple areas of peripheral metastatic disease- in these patients FDG imaging is superior [14]. Combining 11C-acetate and FDG imaging results in the highest overall sensitivity for metastatic disease [14].
11C-choline is incorporated into cells through phosphoylcholine synthesis (and the action of choline kinase) and is integrated into the cell membrane phospholipids [8,13]. 11C-choline has been also been shown to be superior to FDG for the detection of moderately differentiated HCC's (although uptake in poorly differentiated lesions is less than that of FDG) [8]. A drawback of 11C-choline imaging is the short half-life of 11C (about 20 minutes). 18F-fluorocholine has been developed [8]. The agent has low concentration in the normal liver and has also been shown to be superior to FDG for the detection of HCC [8].
|
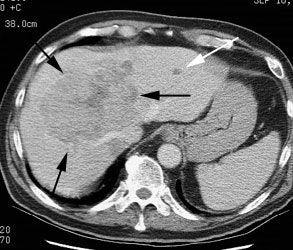
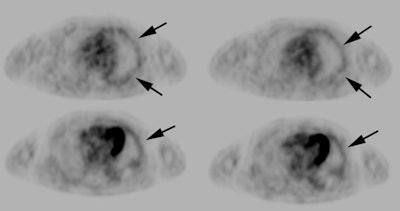
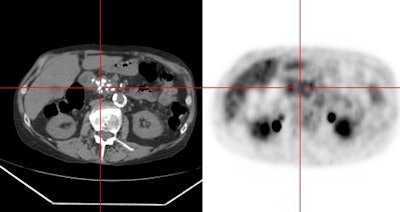
Hepatocellular carcinoma: The patient shown below had a large hepatocellular carcinoma (black arrows). Small satellite lesions were seen in other segments of the liver (white arrow). The FDG PET exam demonstrated only mildly increased uptake of tracer within the patients large primary lesion and the smaller satelite lesions could not be identified. |
|
|
|
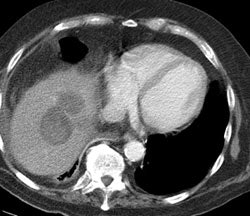
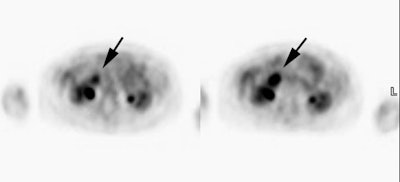
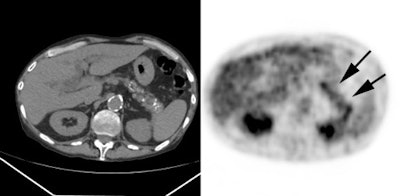
Hepatocellular carcinoma: The patient shown below also has a large hepatocellular carcinoma. The FDG PET exam (right) demonstrated no uptake of tracer within the lesion (black arrows). |
|
|
(1) Liver 2000; Kurtaran A, et al. 18F-fluorodeoxyglucose (FDG)-PET features of focal nodular hyperplasia (FNH) of the liver. 20: 487-490
(2) Radiol Clin N Am 2001; Delbeke D, Martin WH. Positron emission tomography in oncology. 39: 883-917
(3) Radiology 2003; Teefey SA, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR, US, and PET. 226: 533-542
(4) J Nucl Med 2003; Ho CL, et al. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. 44: 213-221
(5) J Nucl Med 2003; Delbeke D. 11C-acetate: a new tracer for the evaluation of hepatocellular carinoma. 44: 222-223
(6) Am Surg 2003; Wudel LJ, et al. The role of [18F]fluorodeoxyglucose positron emission tomography imaging in the evaluation of hepatocellular carcinoma. 69: 117-124
(7) J Nucl Med 2005; Lee JD, et al. Different glucose uptake and glycolytic mechanisms between hepatocellular carcinoma and intrahepatic mass-forming cholangiocarcinoma with increased 18F-FDG uptake. 46: 1753-1759
(8) J Nucl Med 2008; Yamamoto Y, et al. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. 49: 1245-1248
(9) J Nucl Med 2008; Park JW, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. 49: 1912-1921
(10) J Nucl Med 2009; Eckel F, et al. Imaging of proliferation in hepatocellular carcinoma with the in vivo marker 18F-fluorothymidine. 50: 1441-1447
(11) J Nucl Med 2010; Talbot JN, et al. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. 51: 1699-1706
(12) J Nucl Med 2010; Kim HO, et al. Evaluation of metabolic characteristics and viability of lipiodolized hepatocellular carcinomas using 18F-FDG PET/CT. 51: 1849-1856
(13) J Nucl Med 2011; Kuang Y, et al. Imaging lipid syhthesis in hepatocellular carcinoma with [methyl-11C] choline: correlation with in vivo metabolic studies. 52: 98-106
(14) Radiology 2011; PET/CT characteristics of isolated bone metastases in hepatocellular carcinoma. 258: 515-523
(15) Radiology 2000; Katyal S, et al. Extrahepatic metastases of hepatocellular carcinoma. 216: 698-703
(16) Radiographics 2011; Purandare NC, et al. Therapeutic
response to radiofrequency ablation of neoplastic lesions: FDG
PET/CT findings. 31: 201-213
(17) J Nucl Med 2013; Cheung TT, et al. 11C-acetate
and 18F-FDG PET/CT for clinical staging and
selection of patients with hepatocellular carcinoma for liver
transplantation on the basis of Milan criteria: surgeon's
perspective. 54: 192-200
(18) J Nucl Med 2013; Schierz JH, et al. Early dynamic 18F-FDG
PET
to
detect hyperperfusion in hepatocellular carcinoma liver lesions.
54: 848-854
(19) J Nucl Med 2013; Huang WY, et al. 18F-FDG PET
and combined 18F-FDG PET-contrast CT parameters as
predictors of tumor control for hepatocellular carcinoma after
stereotactic ablative radiotherapy. 54: 1710-1716
(20) J Nucl Med 2016; Lee JW, et al. Prognostic significance of
18F-FDG uptake in hepatocellular carcinoma treated
with transarterial chemoembolization or concurrent
chemoradiotherapy: a multicenter retrospective cohort study. 57:
509-516
(21) J Nucl Med 2017; Na SJ, et al. 18F-FDG PET/CT
can predict survival of advanced hepatocellular caricnoma
patients: a multicenter retrospective cohort study. 58: 730-736
(22) Radiology 2017; Chen R, et al.
Fructose-1,6-biphosphonatase 1 reduces 18F FDG
uptake in hepatocellular carcinoma. 284: 844-853
(23) J Nucl Med 2019; Kesler M, et al. 68Ga-labeled
prostate-specific membrane antigen is a novel PET/CT tracer for
imaging of hepatocellular carcinoma: a prospective pilot study.
60: 185-191
Merkel Cell Carcinoma:
Merkel cell carcinoma (MCC) is an aggressive primary cutaneous
neuroendocrine tumor that is a histologic mimic of the family of
small round blue cell tumors [1]. The tumor likely arises from a
dermal pluripotent stem cell with neuroendocrine differentiation
and there may be a viral basis for the tumor (Merkel cell
polyoma virus genome has been detected in MCC) [1]. MCC occurs
in elderly white persons in the 7th-8th decase, affecting men
more than women [1]. Exposure to sunlight, especially
ultraviolet radiation, and immunosuppression are risk factors
for MCC [1]. The risk for developing MCC is 8 times greater in
HIV patients, 25 times greater in patients with organ
transplants, and 40 times greater in those with chronic
lymphocytic leukemia [4].
In decreasing order of incidence the most common sites of MCC
are the head and neck (43%), extremities (15%), and the trunk
(11%) [1]. There is an increased association of MCC and certain
other malignancies such as chronic lymphocytic leukemia,
multiple myeloma, malignancies of the biliary tract, salivary
gland tumors, and other skin malignancies, such as basal cell
carcinoma [1]. There is also increasing evidence of a pathogenic
association with Merkel polyomavirus [3].
Clinically, MCC presents as a rapidly growing firm, non-tender,
dome-shaped red, purple, violet, or skin-colored nodule or less
commonly a plaque-like, nontender cutaneous mass that
infrequently ulcerates [1,4]. Satellite nodules and lymph node
mets are common at presentation (10-45% of patients have LN mets
at presentation) [1]. Another 16-38% have occult nodal
metastases at SLNB [4]. Distant mets can be see in 2-8% of cases
at presentation [1].
There is a high incidence of local recurrence following
treatment - up to 30% of patients within 2 years [1]. Metastases
to LNs are found in more than 50% of cases during the disease
course, and other distant mets are seen in 34-36% of cases- most
commonly to non-regional lymph nodes, the skin or subcutaneous
tissues, lung, liver, brain, and bone [1,4].
Stage I tumors are small than 2 cm in max diameter, stage II
tumors are larger than 2 cm or invade structures such as fascia,
muscle, cartilage, and bone [1]. A positive SLNB increases the
stage of any tumor to stage III (which is defined as
micrometastases - N1a, or macrometastases - N1b in the regional
lymph nodes) [1]. The presence of in-transit mets- defined as
metastatic deposits between the primary tumor and regional lymp
nodes or deposits distal to the primary tumor also upstages a
tumor to stage III (N2) [1]. Stage IV disease is defined as the
presence of distant metastases beyond regional lymph node [1].
The overall five-year survival is 30-64% and this is worse than
for melanoma [1]. The five year survival for stage I disease can
be as high as 75-80%, while survival drops to 20-25% at 5 years
for patients with stage IV disease [1]. Other authors indicate
an overall 5 year survival rate of 51% for local disease, a rate
of 35% in patients with nodal metastases, and 14% in patients
with distant disease [4]. Overall, the most powerful predictor
of survival is the presence or absence of nodal disease [2].
On FDG PET imaging, the primary tumor in MCC has high metabolic
activity [1,2]. FDG PET/CT is also very sensitive (up to 83%)
for detecting region LN mets and is superior to CT alone [1].
However, SLNB remains the cornerstone for the workup of MCC as
micrometastases can be missed on PET/CT imaging [1].
Micrometastases can be detected during lymphoscintigraphy in as
many as 25% of cases of MCC [1].
In-transit mets can also be identified on PET imaging [1].
PET/CT findings can result in upstaging of 50% of clinical stage
I or II MCC to stage III [1]. Other authors indicate that PET
results in upstaging in 17% of patients, and down staging in 5%
[2]. Initial PET exam findings can have an impact on patient
management in 37-40% of patients [2,4]. Restaging PET imaging
after treatment can affect patient management in up to 56% of
cases [3].
A complete metabolic response on PET imaging following therapy
is significantly associated with improved overall survival [3].
Early peritumoral lymphatic invasion can be seen on CT or MR
and appears as subcutaneous reticular fat stranding and
subcutaneous satellite nodules [1].
REFERENCES:
(1) AJR 2013; Merkel cell carcinoma: a primer for the
radiologist. 200: 1186-1196
(2) J Nucl Med 2013; Siva S, et al. 18F-FDG PET
provides high-impact and powerful prognostic stratification in
the staging of Merkel cell carcinoma: a 15-year institutional
experience. 54: 1223-1229
(3) J Nucl Med 2015; Byrne K, et al. 15-year experience of 18F-FDG
PET imaging in response assessment and restaging after
definitive treatment of Merkel cell carcinoma. 56: 1328-1333
(4) Radiographics 2019; Akaike G, et al. Imaging of Merkel cell
carcinoma: what imaging experts should know. 39: 2069-2084
Mesothelioma:
See also discussion on
Mesothelioma in the Chest section for more details.
Malignant pleural mesothelioma accounts for over 90% of primary
pleural malignancies [8]. is associated with asbestos exposure,
however, up to 20% of cases have no relavant asbestos exposure
history [8].
FDG uptake in mesothelioma is significantly greater than in benign pleural disease [3]. Higher FDG uptake in the lesion is also associated with significantly shorter survival [2]. Sensitivity has been reported to be 91-100% and specificity 100%. An occasional false negative result can occur and this is most commonly seen in association with the epitheliod subtye [8]. The role of PET imaging is to aid in documenting the extent of pleural disease, establish mediastinal lymph node involvement, diagnose extrathoracic metastatic disease, and assess treatment response [5,7]. PET imaging is superior to CT imaging for the detection of extrathoracic metastases [4]. Following PET imaging, the stage of of disease can be increased in up to 13% of patients, and decreased in up to 27% [6].
|
Mesothelioma: The patient shown below presented with a complex, loculated left pleural effusion and pleural thickening. Note the presence of an asbestos pleural plaque along the right anterior pleural surface (white arrow). Pleural biopsy revealed mesothelioma. PET scan revealed circumferential mild tracer uptake along the left pleural surface. |
|
|
REFERENCES:
(1) Radiographics 2002; Roach HD, et al. Asbestos: when the dust settles- an imaging review of asbestos-related disease. 22: S167-S184
(2) Radiographics 2004; Wang ZF, et al. Malignant pleural mesothelioma: evaluation with CT, MR imaging, and PET. 24: 105-119
(3) J Nucl Med 2004; Kramer H, et al. PET for the evaluation of pleural thickening observed on CT. 45: 995-998
(4) Radiol Clin N Am 2005; Mavi A, et al. Fluorodeoxyglucose-PET in characterizing solitary pulmonary nodules, assessing pleural diseases, and the initial staging, restaging, therapy planning, and monitoring response of lung cancer. 43: 1-21
(5) Radiology 2006; von Schulthess GK, et al. Integretated PET/CT: current applications and future directions. 238: 405-422
(6) J Nucl Med 2006; Bunyaviroch T, Coleman RE. PET evaluation of lung cancer. 47: 451-469
(7) J Nucl Med 2007; Francis RJ, et al. Early prediction of
response to chemotherapy and survival in malignant pleural
mesothelioma using a novel semiautomated 3-dimensional
volume-based analysis of serial 18F-FDG PET scans.
48: 1449-1458
(8) AJR 2012; Makis W, et al. Spectrum of malignant pleural and
pericardial disease on FDG PET/CT. 198: 678-685
Multiple Myeloma:
Multiple myeloma (MM) is a malignant hematologic disorder
characterized by bone marrow infiltration with neoplastic plasma
cells [4,7,17]. It is the most common primary osseous malignancy
and the second most common hematologic malignancy after
non-Hodgkin lymphoma [4,7,17,18]. MM has three potential
components- diffuse marrow infiltration, focal bone lesions
without marrow infiltration (plasmacytoma), and soft-tissue
(extra-medullary) disease [7]. Extra-medullary disease should be
differentiated from "breakout" lesions that represent sites
where the tumor has broken through the cortex into the
surrounding soft tissues [7].
MM typically evolves from an asymptomatic, premalignant condition called monoclonal gammopathy of undetermined significance (MGUS) [7]. MGUS is defined as a monoclonal expansion of plasma cells with less than 10% infiltration of the bone marrow by monoclonal plasma cells, a serum monoclonal M-protein level of less than 30 g/L (3g/dL), and no end-organ damage [7,10,12]. Approximately 3-4% of the population older than age 50 years has MGUS and in approximately 20% of these cases will progress to myeloma [17]. The rate of progression to MM (or some other B-cell or lymphoid cancer) is approximately 1% per year [12]. MGUS eventually progresses to "smoldering MM" when a bone marrow biopsy shows a 10-60% diffuse infiltration of plasma cells [7]. Smoldering MM is an intermediate premalignant stage with a mean rate of progression to myeloma of 10% per year over the first 5 years [17]. When bone marrow infiltration exceeds 60% or if end-organ damage is present, the patient is diagnosed with active MM [7]. The risk of progression from smoldering MM is 10% per year for the first 5 years [12].
The malignant plasma cells in MM can produce several types of
immunoglobulin-heavy chains with an accompanying light chain;
the most common types of heavy chains are IgG (52%) and IgA
(21%) with kappa as the predominant light chain [9]. MM is
typically characterized by the secretion of a monoclonal protein
(M-protein) which is detected in the blood or urine [7].
However, in 1-5% of patients the disease is classified as hypo-
or nonsecretory [7,9]. Most of these patients will have elevated
kappa or gamma light chains (ligh chain fragments of
immunoglobulins), so the true incidence of nonsecretory MM is
less than 1% [7]. Measuring treatment response in nonsecretory
patients is difficult as serial M-protein or free light chains
cannot be followed [7]. PET or MRI is most useful in these cases
[7].
The majority of myeloma tumors will have cytogenic
abnormalities [9]. The most common chromosomal abnormalities
include chromosome 13q14 deletion (del13q14), chromosome 1q21
gain (amplq21), and chromosome 17p13 deletion (del17p13) [9].
The deletion of chromosome 13q is present in up to 50% of
patients and is generally a negative prognostic factor for
patients receiveing chemotherapy or autologous stem cell
transplant [9]. Amplification of 1q21 is present in about 40% of
patients and it is also a negative prognostic factor [9]. The
17p13 deletion is found in 20% of patients and these patients
show the lowest level of response to therapy [9].
Median age at diagnosis is 69 years (60-70 years), however, 10%
of affected patients are younger than 50 years and 2% are below
age 40 [7,9,17,18]. Males are affected more than females and the
disease is more prevalent among African Americans than among
those of European ancestry [6]. Anemia is the most common
presenting symptom (73% of patients at time of diagnosis),
followed by bone pain, elevated creatinine, fatigue,
hypercalcemia, and weight loss [9]. Approximately 5-10% of
patients present with a solitary plasmacytoma and two-thirds of
these patients will progress to multiple myeloma- presumably the
result of occult disease present at the time of initial
diagnosis [4]. In fact, MR imaging of the spine can detect
additional lesions in one-third of patients considered to have a
solitary plasmacytoma [4]. The presence and extent of bone
marrow and extramedullary involvement are important factors
which influence prognosis and clinical management [4].
Patients with Stage I myeloma (only limited alteration in blood
parameters and not more than one skeletal lesion) may be
followed clinically without therapy [4]. Patients with Stage II
or Stage III myeloma require chemotherapy [4]. Improved survival
has been seen in association with stem cell transplant in
conjunction with therapeutic agents such as lenalidomide,
thalidomide, and bortezomib [6]. The 5-year survival rate is
45-52% [9,18]. The 10 year survival for patients presenting at
an age below 60 years is approximately 30% [7]. The following
features define a complete response to therapy- a CBMPC
percentage lower than 5%, undetectable monoclonal protein in the
serum and urine, and the disappearance of any soft-tissue
plasmacytomas [17].
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy,
presence of M-protein, and skin changes- also known as Takatsuki
syndrome) is a rare paraneoplastic syndrome caused by an
underlying plasma cell disorder that demonstrates osteosclerotic
bone lesions [7,13]. Lymphadenopathy- especially Castleman
disease, is another hallmark of POEMS syndrome [13]. Patients
with POEMS syndrome typically have 5% or less clonal plasma
cells on their bone marrow biopsy specimen [13]. POEMS syndrome
patients have a better median survival compared to classic MM
patients [7]. Bone lesions can be seen in up to 95% of patients
[13]. The sclerotic bone lesions seen in POEMS syndrome may not
be evident on FDG PET imaging (lesions identified on FDG PET
imaging typically have a lytic component [13]).
Extramedullary myeloma is seen in 7-20% of newly diagnosed
myeloma and in 6-20% of cases during the disease course [11,15].
In up to 45% of patients with extramedullary myeloma, the tumor
develops at the time of relapse, particularly in patients with
allogenic bone marrow transplantation [11]. The presence of
extramedullary lesions has been shown to be associated with a
poor prognosis and requires different treatment strategies [15].
Thalidomide has been shown to have poor efficacy in patients
with extramedullary lesions, even when the intramedullary lesion
shows response [15]. Bortezomib has been shown to be an
effective agent in MM patients with extra-medullary lesions, and
high-dose therapy with autologous stem cell transplant can
overcome the negative prognostic impact in selected younger
patients [15].
Lesion detection:
Plain film radiographs are generally obtained for staging, but they can significantly underestimate the extent and magnitude of bone and bone marrow involvement in patients with multiple myeloma (at least 50% focal bone loss must occur in a vertebral body or a lytic lesion to be seen on plain film [7]) [1]. Whole-body low dose CT will reveal lytic osseous lesions in 20-25% of patients with an initial negative skeletal survey [18]. Bone scanning is also inadequate for the detection of myelomatous bone lesions due to the minimal osteoblastic response [4].
FDG PET imaging can detect bone marrow involvement and has been
shown to be useful in assessing disease extent and response to
therapy [4]. PET imaging has the advantage of providing a whole
body survey to assess for disease [4]. FDG PET imaging can
detect lesions not evident on plain film radiographs [3]. PET
imaging has been shown to be superior to conventional skeletal
surveys for the detection of myeloma, with the exception of the
skull and ribs [12].
FDG PET exams are positive in 93% to 100% of patients with
myeloma- showing both focal and diffuse osseous involvement
[1,2,3]. Reported sensitivity for detection of myelomatous
involvement is 59-85% and specificity 75-92% [4,12]. FDG PET
imaging will reveal a greater extent of disease in 25% to 61% of
patients than routine radiographs [1,3] and another 25% will
have unsuspected focal extramedullary disease identified on PET
imaging [1].
However, false-negative exams have been reported in up to 11% of patients (despite evidence of disease on diffusion-weighted MRI) and this has been suggested to be linked to low hexokinase 2 (HK2) gene expression (although this has not be confirmed consistently and there are likely other causes which have not yet been identified) [14]. False negative exams can also be seen in association with subcentimeter sized lesions, skull lesions, diffuse infiltrative disease, and occasionally larger lesions may show only mild increased FDG uptake [4,6,9,12,17]. False positive exams can be seen in association with inflammatory changes associated with radiation therapy or surgery [4]. Therefore, PET imaging is best performed at least 2 months following radiation therapy [4].
It is important that corticosteroid treatment be suspended for at least 5 days before PET imaging, since the administration of steroids may result in false-negative findings [6].
In patients with monoclonal gammopathy of undetermine
significance (MGUS) a negative FDG PET exam reliably predicts
stable disease [1]. Development of myeloma in these patients is
coincident with positive findings on PET imaging [1].
Studies have suggested that whole-body DWI MRI is more
sensitive than PET imaging for the detection of intra-medullary
myeloma lesions [15].
Management:
Clinical management can be influenced in up to 14% of patients
as a result of findings on the PET scan [3]. In the National
Oncologic PET registry, patient management was changed in almost
49% of patients based on the FDG PET scan results [9]. Restaging
of MM patients with FDG PET is best done 2 and preferably 4 or
more weeks after treatment cycle completion [7]. However, if
progression or relapse while on treatment is suspected, PET can
provide verification and direct biopsy [7].
Prognosis:
FDG imaging findings also contain prognostic information [1,7].
A baseline SUVmax of greater than 4.0 is associated with a
shorter progression-free survival [9]. The presence of 3 or more
focal lesions at baseline PET (or 7 or more on MRI) is
associated with a worse prognosis (other authors indicate that
MORE than 3 FDG avid lesions is associated with a 30-month event
free survival of 87% versus 66% [9]) [7]. Another study
concluded that the presence of more than 3 lesions in the
appendicular skeleton on baseline pre-treatment PET was
typically associated with more aggressive forms of myeloma and
was an independent predictor of shorter progression free and
overall survival, compared to patients with no appendicular
skeletal lesions [16]. This study also noted that patients with
appendicular skeletal lesions on MDCT without high FDG uptake
also showed poorer outcomes [16]. The presence of extramedullary
disease is associated with a poor prognosis both before
treatment and with disease relapse [1,7,9].
Complete normalization of FDG PET uptake prior to autologous
stem cell transplantation is correlated with better overall and
event free survival (89% 30-month event free survival in
patients with complete normalization versus 63% in patients with
residual FDG avid lesions) [9]. Persistent positive FDG PET
exams after induction therapy also predicts early relapse [1].
Though an increase in focal lesion size indicates progression of
disease, it is the number of focal lesion, not the size, that
correlates with poor patient outcome [7]. PET imaging can also
be used to determine the total metabolic tumor burden (which
takes into account the volume of all active MM lesions) and this
has also been shown to correlate with progression-free and
overall survival [8].
Other agents in multiple myeloma:
11C-acetate PET/CT has also studied in patients with MM [10]. In patients with MM, de novo lipid synthesis is elevated in the patients due to increased membrane phospholipid synthesis in proliferating plasma cells [10]. 11C-acetate PET/CT been shown to have better lesion detection than FDG PET/CT in patients with multiple myeloma [10]. The agent can also provide risk stratification and the exam is negative in patients with MGUS and indolent/smoldering MM [10].
REFERENCES:
(1) J Nucl Med 2002; Durie BG, et al. Whole-body 18F-FDG
PET
identifies
high-risk myeloma. 43: 1457-1463
(2) Radiology 2004; Angtuaco EJ, et al. Multiple myeloma: clinical review and diagnostic imaging. 231: 11-23
(3) Eur J Nucl Med Mol Imaging 2002; Schirrmeister H, et al. Initial results in the assessment of multiple myeloma using 18F-FDG PET. 29: 361-366
(4) AJR 2005; Bredella MA, et al. Value of FDG PET in the assessment of patients with multiple myeloma. 184: 1199-1204
(5) AJR 2009; Shortt CP, et al. Whole-body MRI versus PET in assessment of multiple myeloma disease activity. 192: 980-986
(6) Radiographics 2010; Hanrahan CJ, et al. Current concepts in
the evaluation of multiple myeloma with MR imaging and FDG
PET/CT. 30: 127-142
(7) J Nucl Med 2012; Walker RC, et al. Imaging of multiple
myeloma and related plasma cell dyscrasias. 53: 1091-1101
(8) J Nucl Med 2012; Fonti R, et al. Metabolic tumor volume
assessed by 18F-FDG PET/CT for the prediction of
outcome in patients with multiple myeloma. 53: 1829-1835
(9) AJR 2013; Agarwal A, et al. Evolving role of FDG PET/CT in
multiple myeloma imaging and management. 200: 884-890
(10) J Nucl Med 2014; Ho C, et al. 11C-acetate
PET/CT for metabolic characterization of multiple myeloma: a
comparative study with 18F-FDG PET/CT. 55: 749-752
(11) AJR 2014; Tirumani SH, et al. MRI features of extramedullary myeloma. 202: 803-810
(12) Radiographics 2015; Ferraro R, et al. MR imaging and
PET/CT in diagnosis and management of multiple myeloma. 35:
438-454
(13) J Nucl Med 2015; Pan Q, et al. Characterizing POEMS
syndrome with 18F-FDG PET/CT. 56: 1334-1337
(14) J Nucl Med 2019; Kircher S, et al. Hexokinase-2 expression
in 11C-methionine-positive, 18F-FDG-negative
multiple myeloma. 60: 348-352
(15) AJR 2019; Chen J, et al. Comparison of whole-body DWI and
18F-FDG PET/CT for detecting intramedullary and
extramedullary lesions in multiple myeloma. 213: 514-523
(16) AJR 2019; Abe Y, et al. Medullary abnormalities in
appendicular skeletons detected with 18F-FDG PET/CT
predict an unfavorable prognosis in newly diagnosed multiple
myeloma patients with high-risk factors. 213: 918-924
(17) Radiographics 2019; Ormond Filho AG, et al. Whole-body
imaging of multiple myeloma: diagnostic criteria. 39: 1077-1097
(18) AJR 2020; Marshall C, et al. The role of imaging and
systemic treatments in myeloma: a primer for radiologists. 214:
1321-1334
Myeloid sarcoma:
Myeloid sarcoma is also known as a chloroma or granulocytic
sarcoma and represents a rare extramedullary anifestation of
acute myeloid leukemia (it occurs in 3-5% of AML cases) [1]. Up
to 21% of cases of myeloid sarcoma presented as relapse
following allogenic bone marrow transplantation [1]. The most
common sites of involvement are the bones, lymph nodes, soft
tissues, skin, and breast (breast involvement is typically
bilateral) [1].
TThe lesion has been found to have moderate increased uptake on
FDG PET imaging (SUVmax 2.6-9.7) [1].
REFERENCES:
(1) AJR 2012; Lee EYP, et al. Utility of FDG PET/CT in the
assessment of myeloid sarcoma. 198: 1175-1179
Neuroblastoma-
click here
Nasopharyngeal carcinoma:
Nasopharyngeal carcinoma (NPC) is a relatively uncommon
malignancy with a high frequency in southern China and southeast
Asia [2]. It accounts for only 0.25% of all malignancies in the
US, but accounts for 15-18% of malignancies in Southern China
and 10-20% of childhood malignancies in Africa (pediatric NPC is
usually poorly differentiated) [7]. The male to female ratio is
3:1 [7]. It is most common among patients 40-60 years of age,
with a bimodeal age peak in the 2nd and 6th decades [7].
Infection with the Ebstein-Barr virus is a major risk factor
[6]. In China, nitrosamine-rich salted foods are also considered
a risk factor [7]. Patients often present with local symptoms
such as nasal congestion and epistaxis [7]. However, the
nasopharynx is a relatively clinically silent area, and the
first presentation can be with cervical or distant metastatic
disease [7]. Lateral retropharyngeal nodes are among the most
common sites of nodal spread from NPC [7]. The presence of
cervical node necrosis is a negative prognostic indicator with
overall decreased survival and a greater risk for distant
metastatic disease [10]. Distant metastases are found in 5-41%
of patients- most commonly to bone, lung, and liver [7].
Patients with tumors extending into the parapharyngeal space and
retropharyngeal space have a higher risk of distant metastases
[7].
There are three histologic subtypes: 1- Kerantinizing squamous
cell carcinoma (type 1) is found more often in nonendemic areas,
is associated with smoking, and carries the worst progosis [7];
2- Nonkeratinizing carcinoma (type 2) is radiosensitive and has
a better prognosis [7]; 3- Undifferentiated carcinoma (type 3)
is also radiosensitivie with a good prognosis [7]. In North
America, about 25% of patients with NPC have type 1, 12% have
type 2, and 63% have type 3 [7]. In China, 2% have type 1, 3%
have type 2, and 95% have type 3 [7].
The tumor is very radiosensitive and the overall survival rate for patients with NPC is high (up to 70% at 5 years), however, between 7-13% of patients have residual disease following treatment [6,8]. Induction chemotherapy with 5-FU cispatin is sometimes combined with XRT [7]. For patients with distant metastases the 1 year survival is only about 10% [2]. Therefore, the identification of distant metastases will significantly impact on patient prognosis. About 5% of patients have distant metastases at time of presentation and up to 30% have distant recurrence after primary definitive therapy [2].
FDG PET imaging provides a whole body survey to assess for the
presence of metastatic disease. Mediastinal lymph nodes are the
most common site of unsuspected metastatic disease, followed by
the lung, liver, and bone [2]. Regional lymph nodes metastases
can be found in up to 12% of patients with negative MRI findings
[4]. Using FDG PET, unsuspected distant metastases can be
identified in up to 11-13% of NPC patients with an initial stage
of M0 after conventional imaging evaluation [2,4]. FDG PET has
been shown to be more sensitive than skeletal scinitgraphy for
detecting bone metastases from nasopharyngeal carcinoma [5,8].
FDG PET has been shown to be superior to conventional imaging
for primary M-staging [5]. The presence of heterogeneous FDG
uptake within the tumor correlates with tumor aggressiveness and
poor patient outcome [9].
Recurrence:
In the evaluation of residual/recurrent/metastatic
nasopharyngeal carcinoma, FDG PET has a sensitivity of 89.5-92%,
a specificity of 55.6-90%, an accuracy of 86-92% (overall
restaging accuracy of 73% [6]), a PPV of 90%, and a NPV of
86-91% [1,3,6]. For the evaluation of residual or recurrent
nasopharyngeal cancer following radiotherapy, a meta-analysis
found a pooled sensitivity of 93% and a specificity of 87%
[11].
PET imaging can detect metastatic disease to supraclavicular lymph nodes and distant sites that can be missed on MR imaging [6]. The findings on the PET exam can add significant information for patients with questionable tumor recurrence on MR imaging [1]. Additionally, patients with positive PET exams have an overall worse prognosis compared to PET negative patients [3]. False-positive exams can occur in association with inflammation, infection, and lymphoid hyperplasia [1]. False-negative exams can be seen with small volume or mucosal lesions [1]. Combined use of MR and PET imaging results in the most accurate tumor restaging [6].
(1) J Nucl Med 2004; Shu-Hang N, et al. Clinical usefulness of 18F-FDG PET in nasopharyngeal carcinoma patients with questionable MRI findings for recurrence. 45: 1669-1676
(2) J Nucl Med 2005; Yen TC, et al. The value of 18F-FDG PET in the detection of stage M0 carcinoma of the nasopharynx. 46: 405-410
(3) J Nucl Med 2005; Yen RF, et al. Whole-body 18F-FDG PET in recurrent or metastatic nasopharyngeal carcinoma. 46: 770-774
(4) J Nucl Med 2006; Chan SC, et al. Differential roles of 18F-FDG PET in patients with locoregional advanced nasopharyngeal carcinoma after primary curative therapy: response evaluation and impact on management. 47: 1447-1454
(5) J Nucl Med 2007; Liu FY, et al. 18F-FDG PET can replace conventional work-up in primary M staging of nonkeratinizing nasopharyngeal carcinoma. 48: 1614-1619
(6) Radiology 2008; Comoretto M, et al. Detection and restaging
of residual and/or recurrent nasopharyngeal carcinoma after
chemotherapy and radiation therapy: comparison of MR imaging and
FDG PET/CT. 249: 203-211
(7) AJR 2012; Razek AA, King A. MRI and CT of nasopharyngeal
carcinoma. 198: 11-18
(8) J Nucl Med 2012; Chang KP, et al. Prognostic significance
of 18F-FDG PET parameters and plasma Epstine-Barr
virus DNA load in patients with nasopahryngeal carcinoma. 53:
21-28
(9) AJR 2012; Huang B, et al. Nasopharyngeal carcinoma:
investigation of intratumoral heterogeneity with FDG PET/CT.
199: 169-174
(10) Radiology 2015; Lan M, et al. Prognostic value of cervical
node necrosis in nasopharyngeal carcinoma: analysis of 1800
patients with positive cervical nodal metastasis at MR imaging.
276: 536-544
(11) J Nucl Med 2016; Zhou H, et al. 18F-FDG PET/CT for the diagnosis of residual or recurrent nasopharyngeal carcinoma after radiotherapy: a metaanalysis. 57: 342-347
Pancreatic cancer:
The majority of pancreatic cancers are exocrine- adenocarciomas
(85%) [9]. Pancreatic cancer has a dismal prognosis with a 5
year survival rate of 6% (the survival rate is only 15% in stage
I patients and less than 1% in stage IV patients [10]) [9]. This
is because the majority of patients with pancreatic
adenocarcinoma present with unresectable disease (only 10-20% of
patients with pancreatic adenocarcinoma are deemed surgically
resectable [8]. Even following surgery, 72-92% of patients will
recur locally within 2 years [8] and the overall survival rate
among patients with localized resectable disease is only 23%
[9].
Patients may present with obstructive jaundice, weight loss,
abdominal or midback pain, or a combinations of these symptoms
[9]. New onset glucose intolerance can also be a sign of
panceatic cancer [9]. Trousseau syndrome (migratory
thrombophlebitis) is a well-established paraneoplastic syndrome
associated with panceatic carcinoma [9]. Pancreatic
panniculitis- or subcutaneous areas of nodular fat necrosis, can
also be seen in association with pancreatic cancer (typically
acinar carcinoma) [9]. The panniculitis usually involves the
lower extremities, but can also occur in the buttocks, trunk,
and arms [9].
CT has some limitations for the evaluaiton of pancreatic
adenocarcinoma [8]. The sensitivity of CT is lower for lesions
less than 2 cm (83%) and about 10% of pancreatic cancers are
isoattenuating on post contrast CT imaging [8]. Also- for lymph
node staing, CT has a sensitivity of 37% and a specificity of
79% [8].
On FDG PET imaging most pancreatic cancers show intense, focal
FDG accumulation [1]. Reported sensitivities range from 71% to
96%, and specificity from 61% to 88% (median specificity of 82%)
[1,2,3]. The sensitivity is improved through the use of CT image
fusion [3]. Also- the sensitivity is higher in euglycemic
patients compared to those with elevated glucose levels (83-86%
versus 42-69%) [8].
Prognosis: Patients with higher SUV's in their pancreatic tumor
are have been shown to have a worse prognosis [2,9]. The
metabolic tumor volume and the total lesion glycolysis have also
been shown to be independent predictors of recurrence free and
overall survival [10,11].
False-negative exams can be seen with hyperglycemia or small,
well-defined tumors [1].
|
Pancreatic cancer: The patient shown below had a history of thyroid cancer and was being evaluated for a large neck mass. The PET scan revealed intense uptake in the abdomen anterior to the right kidney (black arrows). Subsequent CT imaging revealed a large mass in the pancreatic head (white arrows). There is atrophy of the remainder of the pancreas and dilatation of the pancreatic duct. |
|
|
False-positive exams can be seen in association with
inflammatory pancreatic processes such as chronic active
pancreatitis and autoimmune pancreatitis can demonstrate FDG
uptake [2]. FDG uptake in chronic pancreatitis is reported to be
uncommon (about 13% of patients[7]) and tends to be relatively
low with an SUV of less than 2.1 and is also more diffuse [2].
Chronic pancreatitis can sometimes be focal and appear mass-like
(mass forming pancreatitis) amking distinction from pancreatic
carcinoma difficult [8]. Patients with MFP are at an increased
risk for adenocarcinoma (20% lifetime risk by the age of 60
years) [8]. In general, MFP will demonstrate only low level
tracer uptake [8]. An SUV max of more than 2.0 carries a
sensitivity of 86-100% for malignancy (but the specificity is
lower 76-77%). An SUV max of over 4.0 has been shown to have a
PPV of 100% for malignancy (and an NPV of 94%) [8].
Similarly, patients with autoimmune pancreatitis commonly show diffuse pancreatic uptake, but have higher SUV max values [7]. Patients with autoimmune pancreatitis may also demonstrate tracer uptake within the salivary glands [7]. Some authors recommend using an SUV of 3.5 as the best cutoff point for differentiating between benign and malignant pancreatic lesions [3].
|
Chronic pancreatitis: The patient below underwent PET imaging for evaluation of a left upper lung nodule. Incidental note was made of diffuse pancreatic tracer accumulation (cross hair and black arrows). Coregistered CT imaging revealed findings consistent with chronic pancreatitis. |
|
|
Metastatic disease: The sensitivity for the detection of lymph
node metastases is about 61% [2], although detections rates as
low as 26% have been observed [3]. The poor detection rate of
lymph nodes metastases in some cases is likely related to their
small size and a peripancreatic location which can limit
differentiation from the primary tumor [3]. PET/MR with the
addition of DW imaging has been shown to increase the
sensitivity for detection of lymph node metastases to 40% from
10% by PET/CT [12].
PET imaging is superior to CT for the detection of distant metastatic disease [3]. However, it may be less sensitive for the detection of liver metastases compared to contrast enhanced CT- in one study 54% of liver metastases were negative on PET imaging [5].
PET findings can significantly impact on patient management in
up to 43% of cases [4]. Up to 17% of patients felt to have
resectable disease are upstaged at PET imaging, thereby avoiding
unnecessary laparotomy with significant cost savings [2,6].
Contrast enhanced PET/CT imaging has been shown to be superior
to PET alone for the evaluation of pancreatic cancer [5].
Tumor recurrence: PET/CT has been shown to be superior to CT
and MRI for the detection of tumor recurrence [8]. On
post-surgical followup, FDG uptake in the surgical bed 3 months
after surgery is usually indicative of recurrence [8].
Other PET agents:
18F-FLT has also been shown to accumulate in pancreatic neoplasms (sensitivity 71%), while it shows little uptake in chronic pancreatitis [6]. As with FDG, small and low grade lesions may not been identified on FLT imaging [6]. Also- normal hepatic FLT activity may obscure metastatic foci to the liver [6].
Increased tracer uptake can also be seen in malignant cystic pancreatic neoplasms [2].
(1) AJR 2003; Tamm EP, et al. Diagnosis, staging, and surveillance of pancreatic cancer. 180: 1311-1323
(2) AJR 2003; Kalra MK, et al. Correlation of positron emission tomography and CT in evaluating pancreatic tumors: technical and clinical implications. 181: 387-393
(3) J Nucl Med 2004; Lemke AJ, et al. Retrospective digital image fusion of multidetector CT and 18F-FDG PET: clinical value in pancreatic lesions - a prospective study with 104 patients. 45: 1279-1286
(4) Radiol Clin N Am 2004; Hustinx R. PET imaging in assessing gastrointestinal tumors. 42: 1123-1139
(5) J Nucl Med 2008; Strobel K, et al. Contrast-enhanced 18F-FDG PET/CT: 1-stop-shop imaging for assessing the resectability of pancreatic cancer: 49: 1408-1413
(6) J Nucl Med 2008; Herrmann K, et al. In vivo characterization of proliferation for discriminating cancer from pancreatic pseudotumors. 49: 1437-1444
(7) AJR 2009; Lee TY, et al. Utility of 18F-FDG
PET/CT for differentiation of autoimmune pancreatitis with
atypical imaging findings from pancreatic cancer. 193: 343-348
(8) Radiographics 2012; Sahani DV, et al. State-of-the-art
PET/CT of the pancreas: current role and emerging indications.
32: 1133-1158
(9) AJR 2012; Dibble EH, et al. PET/CT of cancer patients: part
I, pancreatic neoplasms. 199: 952-967
(10) J Nucl Med 2014; Lee JW, et al. Prognostic value of
metabolic tumor volume and total lesion glycolysis on
preoperative 18F-FDG PET/CT in patients with
pancreatic cancer. 55: 898-904
(11) AJR 2015; Chirindel A, et al. Prognostic value of FDG
PET/CT-derived parameters in pancreatic adenocarcinoma at
initial PET/CT staging. 204: 1093-1099
(12) Radiology 2017; Joo I, et al. Preoperative assessment of
pancreatic cancer with FDG PET/MR imaging versus FDG PET/CT plus
contrast-enhanced multidetector CT: a prospective preliminary
study.282: 149-159
Paraganglioma:
Paragangliomas are most commonly located in the head and neck region [2]. Head and neck paragangliomas (HNPs) are derived from the parasympathetic nervous system and can be present in a variety of locations including the carotid body (60%), vagus nerve (glomus vagale- 13%), jugular bulb (glomus jugulare- 23%), amd tympanic branhc of the ascending phayngeal artery (glumus tympanicum - 6%) [3,5]. HNPs are usually benign, but between 6-19% are malignant [3]. In contrast to paragangliomas at other sites, only 1-3% of HNPs secrete catecholamines (biochemically silent)- therefore, MIBG imaging has a low sensitivity for these tumors [3,5]. About 90% of HNPs are sporadic, whereas the remaining are familial [3]. The latter are important because they are often multicentric and associated with paragangliomas at other sites [3]. Hereditary head and neck paragangiomas are associated with mutations involving succinate dehydrogenase complex sub-units B, C, or D (SDHB, SDHC, SDHD) [5]. Patients with hereditary tumors are at a high risk for metastatic disease (particularly those with SDHB mutations) or prone to developing multiple lesions (particularly those with SDHD mutations) [5].FDG:
Most paragangliomas are FDG avid, despite the fact that they are generally indolent neoplasms [1]. In one study, paragangliomas associated with succinate dehydrogenase (SDHx) mutations (FDG PET sensitivity 71-90.5% [5]) and those associated with von-Hippel Lindau were the most FDG avid tumors [1,5]. There is typically low uptake in RET/NF1 related disease [4].
18F-dihydroxyphenylalanine (DOPA) imaging:
Paragangliomas can also decarboxylate amino acids (APUD tumors) [1]. The agent 18F-FDOPA can also be used to image paragangliomas- especially SDHB negative patients and patients with head and neck paragangliomas [1]. Although other authors suggest that the sensitivity of the agent is only slightly decreased for paraganglioma detection in patients with SDHB mutations [2].
68Ga-DOTANOC/ 68Ga-DOTATATE:
Paragangliomas overexpress somatostatin receptors- mostly SSTR2 [5].
68Ga-DOTANOC is a somatostatin receptor agent that has affinity for SSTR 2-5 (compared to 68Ga-DOTATATE that has affinity for SSTR 2 only) [3]. Both 68Ga-DOTATATE and 68Ga-DOTANOC had excellent sensitivity (up to 100%) for the detection of HNPs [3]. In a review of 68Ga-DOTA agents for the detection of paragangliomas and pheochromocytomas the pooled detection rate was 93%, which is superior to other functional imaging modalities (note- a greater proportion of head and neck paragangliomas was significantly associated with higher 68Ga-DOTA PET detection rates) [6]. Note however, that the detection rate can be much lower in patients with polycythemia/paraganglioma syndrome (35% for 68Ga-DOTA whereas 18F-DOPA exhibits the highest detection for this condition) [6].
The major utility of 68Ga-DOTA imaging is the ability to demonstrate synchronous paragangliomas at other sites and in the detection of metastatic disease [3].
REFERENCES:
(1) J Nucl Med 2012; Taieb D, et al. Modern nuclear imaging for paragangliomas: beyond SPECT. 53: 264-27
(2) J Nucl Med 2012; Rischke HC, et al. Correlation of the Genotype of Paragangliomas and Pheochromocytomas with Their Metabolic Phenotype on 3,4-Dihydroxy-6-18F-Fluoro-l-Phenylalanin PET. 53: 1352-1358
(3) J Nucl Med 2013; 68Ga-DOTANOC PET/CT for baseline evaluation of patients with head and neck paraganglioma. 54: 841-847
(4) Radiographics 2015; Hofman MS, et al. Somatostatin receptor imaging with 68Ga-DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpretation. 35: 500-516
(5) J Nucl Med 2016; Janssen I, et al. 68Ga-DOTATATE PET/CT in the localization of head and neck paragangliomas compared with other functioning imaging modalities and CT/MRI. 57: 186-191
(6) J Nucl Med 2019; Han S, et al. Performance of 68Ga-DOTA-conjugated somatostatin receptor-targeting peptide PET in detection of pheochromocytoma and paraganglioma: a systemic review. 60: 369-376
Pheochromocytoma:
See also the discussion of pheochromocytoma in the Genitourinary Imaging sectionPheochromocytomas and extraadrenal paragangliomas are rare
tumors of neuroectodermal origin that arise from sympathetic and
parasympathetic paraganglia [9,12]. Pheochromocytomas are
catecholamine producing neuroendocrine tumors. About 90% are
solitary and occur in the adrenal gland. The diagnosis of
pheochromocytoma is established by measuring urinary and plama
catecholamines and their metabolites (metanephrine) [2].
Paragangliomas are most commonly located in the head and neck
region and they usually do not secrete catecholamines [12].
Between 25-40% of patients with pheochromocytomas or
paragangliomas have germline mutations of certain susceptibility
genes: RET, von Hippel Lindau, succinate dehydrogenase B to D
(SDHx), or neurofibromatosis type I [12,16]. These mutations are
correlated to various biologic properties of the tumors [12] and
are subdivided into two main clusters [16]. Cluster 1 is
enriched with genes (succinate dehydrogenase complex subunits
A-D [SDHx]) that are associated with hypoxic response [16].
Cluster 2 contains tumors mutated for genes that activate kinase
signaling and protein translatoion (RET protooncogene-MEN2)
[16]. The risk of malignancy in essentially zero in patients
with SDHC and less than 3% in patients with SDHD, however, the
risk increases to about 25% for SDHB mutation carriers [12].
Additionally, the risk for multiple lesions is higher in
patients with VHL or SDHD mutation (55% and 48%, respectively),
compared to NF1 ,SDHB, and SDHC mutations (11-12%) [12].
Other authors divide pheochromocytomas and paragangliomas into
four cluster groups- pseudohypoxia, kinase signaling, Wnt
altered, and cortical admixture [22]. Pseudohypoxia clusters are
further divided into two subtypes- Krebs cycle-related, and VHL
and EPAS1 related [22]. The Krens cycle-related cluster subtype
accounts for 10-15% of pheochromocytomas and paragangliomas, and
the VHL and EPAS1 related subtype accounts for 15-20% [22]. The
Krebs cycle-related subtype includes mutations of the SDH gene
subunits (SDHA, SDHB, SDHC, and SDHD) and other enzymes of the
Krebs cycle [22]. Of these, SDHB and SDHD mutations are more
common and are associated with both abdominal and head and neck
paragangliomas, respectively [22]. Pseudohypoxia pheo's and
paragangliomas with metastatic disease demonstrate a good
response to antiangiogenic therapies such as treatment with
everolimus (a mammalian target of rapamycin inhibitor) and
sunitinib (a potent tyrosine kinase inhibitor) [22].
FDG imaging of pheochromocytomas is limited and the exam has
not been shown to be superior to MIBG imaging (most
paraganglioma lesions demonstrate moderate to low FDG uptake)
[1,21]. Additionally, both benign and malignant
pheochromocytomas and paragangliomas can demonstrate FDG
accumulation [9]. FDG accumulation can be seen in 58% or more of
benign lesions, and 82% or more of malignant pheochromocytomas
[2,9].
FDG uptake is higher in paraganglioma tumors with underlying
succinate dehydrogenase (SDHx) mutations (cluster 1) - likely
related to compromised oxidative phosphorylation and a
pseudohypoxic response which mediates an increase in aerobic
glycolysis [14,16,17]. A more in depth explanation is that SDH
deficiency leads to a truncated tricarboxylic acid cycle with
accumulation of succinate, which through inhibition of prolyl
hydroxylase activity, stabilizes hypoxia-inducible factors
(HIF-alpha isoforms) that upregulate hypoxia-related target
genes, including those involved in glycolytic metabolism
[17,21]. Another factor is related to the extracellular action
of succinate on surrounding stroma cell carbohydrate metabolism
via a hormone-like action [21].
Therefore, FDG avidness does not provide prognostic information
as SDHx tumors may have an indolent course, even when their FDG
uptake is high [16]. SDH mutations also lead to loss of certain
differentiated features such as F-DOPA uptake [17]. VHL-related
tumors can also demonstrate high FDG uptake [14]. (SDHx and
VHL-related tumors show increased uptake at PET imaging compared
to RET and NF-1 related tumors [22])
FDG complements 18F-DOPA in patients with metastatic disease where the metabolic phenotype has shifted to glycolysis (i.e.: patients with a mutation in the succinate dehydrogenase gene B) [10].
18F-dihydroxyphenylalanine (DOPA) imaging:
18F-dihydroxyphenylalanine (DOPA) is an agent
that will be taken up (via monoamine transporter 2),
decarboxylated by amino acid decarboxylase (which is strongly
expressed in tumors of neuroendocrine or neural origin), and
stored by neuroendocrine tumors [1,8,12]. 18F-DOPA
is not taken up by the normal adrenal glands and in a limited
number of patients the agent has shown very high sensitivity for
the detection of pheochromocytomas [1,11].
Imaging is performed one hour following tracer injection
(unlike MIBG imaging that requires 24-48 hours) [8] and can be
performed without the need to withdraw drugs that interfere with
MIBG uptake [11]. The SUV max on 18F-FDOPA PET/CT
correlates with urinary metanephrines and plasma chromogranin A
[21]. In one study, 18F-FDOPA had a sensitivity of
about 85%, specificity of 100%, and accuracy of 92% for the
localization of pheochromocytoma [8]. Other have reported high
diagnostic accuracies between 90-92% [10]. In a meta-analysis,
for detection of pheochromocytoma and paraganglioma, the pooled
lesion based sensitivity was 79% and specificity was 95% [19].
The diagnostic performance of 18F-DOPA is lower for
extra-adrenal paragangliomas and SDHx-related metastatic disease
[19].
Prior to imaging patients receive carbidopa (200 mg) one hour
prior to injection of the radiotracer [8]. Carbidopa- a
peripheral aromatic amino acid decaboxylase inhibitor- decreases
dearboxylation of 18F-DOPA thereby increasing tracer
availability [3]. The agent also appears to block physiologic
tracer uptake in the pancreas [3]. In one study, the use of
carbidopa increased the sensitivity of 18F-DOPA for
the detection of adrenal pheochromocytomas by increasing the
tumor-to-background ratio (from 56% to 67%) [3,8].
18F-fluorodopamine ( 18F-DA)
can also be used for localization of pheochromocytomas [4,5].
The agent has been shown to be superior to I-131 MIBG imaging
for the detection of metastatic disease [5]- this may be due to
a better affinity of 18F-DA for the norepinephrine
membrane transporter system and increased resolution of PET
compared to planar gamma-camera imaging [7]. Reported overall
sensitivity for 18F-DA PET for the detection of
pheochromocytoma is 90% [7]. Unfortunately, 18F-DA
also localizes to the normal adrenal gland and this may lead to
false-positive results [4]. Typically, uptake within
pheochromocytomas is higher than that of normal adrenal tissue
[4]. Other sites of localization of the agent include the liver,
kidneys, heart, thyroid, spleen (faint), parotid glands, and
lung (very faint) [4].
18F-meta-fluorobenzylguanidine (18F-MFBG): 18F-MFBG is an analog of MIBG that can be used to image neuroendocrine tumors, pheochromocytoma, paraganglioma, or neuroblastoma [18]. The dose is 4-12 mCi and imaging is best performed approximately two hours following administration to maximize lesion detection [18]. Normal activity is seen in the genitourinary tract, liver, salivary glands, lacrimal glands, thyroid, heart, prostate, adrenals, and pancreas [18]. There is mild activity in the spleen and GI tract [18]. The agent has been shown to detect more lesions than MIBG scintigraphy [18].
68Ga-DOTA-peptides: 68Ga-DOTA-peptides
can also be used for the detection of pheochromocytoma [15].
SDH-related pheochromocytomas/paragangliomas over-express
somatostatin receptors [20].
In one study, 68Ga-DOTANOC PET/CT had a lesion
based accuracy of 91% compard to 67% for I-131 MIBG [15].
18F-FLT: 18F-FLT has been shown to have low
uptake in pheochromocytomas and paragangliomas [16].
The proposed choice of imaging agent for
pheochromocytomas and paragangliomas can be guided by tumor
location (which is tightly linked to embryologic origin) and
the tumor genetics [20]:
| Condition
|
Imaging agent 1st choice |
Imaging agent 2nd choice |
| Pheochromocytoma (sporadic) |
18F-FDOPA or 123I-MIBG |
68Ga-SSA |
| Inherited pheo (NF1/RET/VHL/MAX) |
18F-FDOPA | 123I-MIBG or 68Ga-SSA |
| Extra-adrenal sympathetic, multifocal or metastatic, or SDHx mutation |
68Ga-SSA | 18F-FDG and 18F-FDOPA |
| HNPGL |
68Ga-SSA | 18F-FDOPA |
123I-MIBG images the
norepinephrine transporter system, 68Ga-SSA
images somatostatin overexpression, and 18F-FDOPA
images dopamine biosynthesis [21].
REFERENCES:
(1) Radiology 2002; Hoegerle S, et al. Pheochromocytomas: detection with 18F DOPA whole-body PET- initial results. 222: 507-512
(2) AJR 2006; Yoon JK, et al. Incidental pheochromocytoma mimicking adrenal adenoma because of rapid contrast enhancement loss. 187: 1309-1311
(3) J Nucl Med 2007; Timmers HJ, et al. The effects of crbiopa on uptakeof 6-18F-fluoro-L-DOPA in PET of pheochromocytoma and extraadrenal abdominal paraganglioma.48: 1599-1606
(4) J Nucl Med 2007; Timmers HJLM, et al. Usefulness of standardized uptake values for distinguihing adrenal glands with pheochromocytoma from normal adrenal glands by use of 6--18F-fluorodopamine PET. 48: 1940-1944
(5) J Clin Endocrinol Metab 2003; Ilias I, et al. Superiority of 6-[18F]-Fluorodopamine positron emission tomography versus [131I]-Metaiodobenzylguanidine scintigraphy in the localization of metastatic pheochromocytoma. 88: 4083-4087
(6) J Nucl Med 2008; Jager PL, et al. 6-L-18F-fluorodihydroxyphenylalanine PET in neuroendocrine tumors: basic aspects and emerging clinical applications. 49: 573-586
(7) J Nucl Med 2008; Ilias I, et al. Comparison of 6-18F-Fluorodopamine PET with 123I-metaiodobenzylguanidine and 111In-pentreotide scintigraphy in localization of nonmetastatic and metastatic pheochromocytoma. 49: 1613-1619
(8) J Nucl Med 2009; Imani F, et al. 18F-FDOPA PET and PET/CT accurately localize pheochromocytomas. 50: 513-519
(9) J Nucl Med 2009; Taieb D, et al. 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? 50: 711-717
(10) J Nucl Med 2009; Minn H, et al. 18F-DOPA: a
multiple-target molecule. 50: 1915-1918
(11) J Nucl Med 2012; Taieb D, et al. Modern nuclear imaging for paragangliomas: beyond SPECT. 53: 264-27
(12) J Nucl Med 2012; Rischke HC, et al. Correlation of the Genotype of Paragangliomas and Pheochromocytomas with Their Metabolic Phenotype on 3,4-Dihydroxy-6-18F-Fluoro-l-Phenylalanin PET. 53: 1352-1358
(13) J Nucl Med 2013; Sharma P, et al. 68Ga-DOTANOC PET/CT for
baseline evaluation of patients with head and neck
paragangliomas. 54: 841-847
(14) J Nucl Med 2014; van Berkel A, et al. Correlation between
in vivo 18F-FDG PET and
immunohistochemical markers of glucose uptake and metabolism
in pheochromocytoma and paraganglioma. 55: 1253-1259
(15) Radiographics 2015; Hofman MS,
et al. Somatostatin receptor imaging with 68Ga-DOTATATE
PET/CT: clinical utility, normal patterns, pearls, and pitfalls
in interpretation. 35: 500-516
(16) J Nucl Med 2015; Blanchet EM, et al. 18F-FLT
PET/CT in the evaluation of pheochromocytomas and
paragangliomas: a pilot study. 56: 1849-1854
(17) J Nucl Med 2017; Ta?eb D, et al. PET
imaging for endocrine malignancies: from woe to go. 58:
878-880
(18) J Nucl Med 2018; Pandit-Taskar N, et
al. Biodistribution and dosimetry of
18F-meta-fluorobenzylguanidine: a first-in-human
PET/CT imaging study of patients with neuroendocrine
malignancies. 59: 147-153
(19) J Nucl Med 2019; Han S, et al. Performance of 68Ga-DOTA-conjugated
somatostatin receptor-targeting peptide PET in detection of
pheochromocytoma and paraganglioma: a systemic review. 60:
369-376
(20) J Nucl Med 2020; Taieb D, Pacak K. Genetic determinants of
pheochromocytoma and paraganglioma imaging phenotypes. 61:
643-645
(21) J Nucl Med 2020; Taied D, et al. Molecular imaging in the
era of precision medicine: paraganglioma as a tmeplate for
understanding mutliple levels of analysis. 61: 646-648
(22) Radiographics 2020; Katabathina V, et al. Decoding genes: current update on radiogenomics of select abdominal malignancies. 40: 1600-1626
Schwannomas typically arise from spinal nerve roots and the cervical, sympathetic, vagus, peroneal, and ulnar nerves in the head, neck, and flexor surfaces of the upper and lower extremities [1]. They commonly present as non-specific masses that are associated with a peripheral nerve [1]. Patients are typically in the 3rd to 5th decade of life. Most lesions are solitary [1]. There is an association with neurofibromatosis type I [1]. Resection is performed for symptomatic or large lesions, or those that demonstrate rapid growth [1].
Schwannomas generally demonstrate increased FDG accumulation with a wide range of SUV values (even greater than 6) [1]. As a result, it is not possible to distinguish schwannomas from malignant peripheral nerve sheath tumors [1].
REFERENCES:
(1) AJR 2004; Beaulieu S, et al. Positron emission tomography of
schwannomas: emphysing its potential in preoperative planning.
182: 971-974
Testicular germ cell tumors:
Through the use of cisplatin-based chemotherapy followed by secondary resection of residual masses, 70-80% of patients with metastatic germ cell tumors can be cured [4].
Initial staging:
For patients with testicular cancer, proper initial staging is very important in order to classify patients into low and high-risk groups [2]. Unfortunately, CT imaging can have a false-negative rate as high as 59% [2]. Based upon conventional staging methods, up to 50% of patients are understaged and 25% are overstaged [2]. In stage I nonsemanomatous GCT approximately 30% of patients will relapse and up to 45% do not have increased tumor markers [3]. The majority of relapses (61%) occur in the para-aortic nodes [3]. Approximately 80% of relapses occur within the first year after surgery and 90% by 2 years [3].
Studies have suggested that PET imaging is superior to CT for staging testicular tumors [2]. FDG PET imaging has been shown to have a sensitivity of 70-87%, specificity 94-100%, PPV 94%, and a NPV of 94% [2]. PET does not improve staging for patients with clinical stage I disease because, if present, metastases tend to be of small volume [3].
Residual masses:
Following treatment for nonseminomatous germ cell tumors (NSGCT), residual tumor lesions can be found in up to 40% of patients despite normalization of serum tumor markers [1]. Approximately 50% of residual lesions consist only of necrotic tissue, about 30% contain mature teratoma (FDG PET is also not able to identify mature teratoma [3]), and only 20% contain residual immature tumor cells. Presently, the only way to confirm lack of any residual tumor is with surgical resection and histologic analysis [1]. Because patients with only necrosis do not benefit from surgical resection of the residual mass, it would be useful to properly identify patients with residual viable tumor. In the evaluation of residual masses for viable tumor FDG PET imaging has been shown to have a sensitivity of 20-88%, a specificity of 92-100%, a PPV of 100%, and a NPV of 48-60% [1,2,4]. Therefore, lack of FDG accumulation does not exclude residual viable tumor cells (PET is unable to accurately identify tumor transformed mature teratoma), but does suggest a low likelihood for residual disease [3,4]. A positive PET scan, however, is highly predictive of the presence of residual viable tumor (positive predictive value of 91-96%) [1,2] although inflammation can produce a false-positive result [3]. Patient's with residual mature differentiated teratoma require surgery because there is a risk of the mass undergoing malignant transformation [3].
Positive tumor markers with negative conventional imaging:
FDG PET may play a role in this setting [3]. A large percentage of patients will be discovered to have sites of disease on PET imaging [3].
REFERENCES:
(1) Cancer 2002; Kollmannsberger C, et al. Prospective comparison of [18F] fluorodeoxyglucose positron emission tomography with conventional assessment by computed tomography scans and serum tumor markers for the evaluation of residual masses in patients with nonseminomatous germ cell carcinoma. 94: 353-62
(2) Radiol Clin N Am 2004; Kumar R, et al. PET in the management of urologic malignancies. 42: 1141-1153
(3) AJR 2008; Sohaib SA, et al. The role of imaging in the diagnosis, staging, and management of testicular cancer. 191: 387-395
(4) J Nucl Med 2010; Pfannenberg C, et al. PET/CT with 18F-FLT: does it improve the therapeutic management of metastatic germ cell tumors? 51: 845-853
Unknown Primary:
The incidence of unkown primary tumors in oncologic patients is 0.5 to 7% at the time of initial diagnosis [2,3]. The primary tumor is detected in 20- 40% of the patients by conventional diagnostic procedures [2,4]. FDG PET (and/or PET/CT) imaging can identify the primary tumor in about 24-57% of patients with negative conventional imaging results [2,3,4]. A meta-analysis has shown PET imaging to have an overall sensitivity of 87% and a specificity of 71% in the detection of the primary tumor [2].
In a subgroup of patients that present with CNS symptoms secondary to an unknown primary, FDG PET imaging can be used to identify the site of the primary lesion with a sensitivity of about 80%, a specificity of 94%, positive predictive value of 75%, and a negative predictive value of 85% [1]. An added benefit of FDG PET imaging is that additional sites of bone or distant metastatic disease can be found in 18% and 31% of patients, respectively [1]. Lack of identification a tumor primary on PET imaging indicates a high likelihood for a primary CNS lesion (specificity 94%) [1].
REFERENCES:
(1) J Nucl Med 2002; Jeong HJ, et al. Usefulness of whole-body 18F-FDG PET in patients with suspected metastatic brain tumors. 43: 1432-1437
(2) J Nucl Med 2003; Delgado-Bolton R, et al. Meta-analysis of the performance of 18F-FDG PET in primary tumor detection in unknown primary tumors. 44: 1301-1314
(3) Radiology 2004; Gutzeit A, et al. Unknown primary tumors: detection with dual-modality PET/CT- initial experience. 234: 227-234
(4) Radiology 2007; Blodgett TM, et al. PET/CT: form and function. 242: 360-385