Pancreatic-Renal Transplant:
Clinical:
Combined kidney-pancreas transplants are done with increasing
frequency in patients with poorly controlled diabetes in an effort
to
restore normal endocrine function of the pancreas and prolong
renal
transplant survival. Pancreatic transplantation is the most
effective
method of achieving tight glucose control and can potentially
stabilize
or reverse complications associated with diabetes [5]. Graft
function
rates following kidney-pancreatic transplantation can be as high
as
84-95% at one year [5,7,9]. At 15 years, the patient and graft
survival
rates are 56% and 36%, respectively [9]. The pancreas is sometimes
transplanted after the patient has previously received a kidney
transplant, but this is associated with a lower 1 year graft
survival
of
78% [9]. The pancreas allograft (with a short segment of duodenum)
is
typically placed on the right side of the abdoen
intraperitoneally,
while the donor kidney is generally placed in the left iliac fossa
[10].
Pancreatic exocrine secretions can be drained to the urinary
bladder
or to the bowel. For urinary bladder drainage, a short segment of
duodenum associated with the pancreatic transplant is anastamosed
to
the dome of the bladder. The advantages of this procedure are a
low
technical complication rate and the ability to monitor graft
rejection
by following urinary amylase levels [5,9]. However, exocrine
secretions in the bladder can
produce cystitis, metabolic acidosis, bladder stones, and graft
pancreatitis due to reflux [5]. About 5-10% of cases will require
a
second operation to convert from bladder to enteric drainage [5].
When
bladder drainage is performed, the venous drainage is to the
systemic
circulation via external iliac vein [10,13], but unfortunately,
the
procedure
directs venous outflow from the transplanted pancreas away from
the
liver and into the systemic circulation which can result in
peripheral
hyperinsulinemia.
Enteric exocrine drainage is used most commonly (82-90%
of simultaneous renal-panc transplants) [9,11]. For enteric
drainage, an
asatomosis is created between the donor duodenal stump and a small
bowel loop, with or without the creation of a Roux-en-Y loop
[9]. For enteric drainage, the head of the pancreas is
placed in the
cephalic position with a side-to-side anastomosis with the the
donor
duodenum draining exocrine secretions into a small-bowel loop [5].
The
pancreatic venous drainage is into the portal venous system via a
transplanted portal vein to recipient superior mesenteric vein
anastomosis [5,9]. Although drainage to the superior mesenteric
vein is "more physiologic" it has not been demonstrated to be
superior to systemic venous drainage [11].
Islet transplantation is an innovative technique for treating
patients with type 1 diabetes [10]. Purified islets of Langerhans
extracted from deceased donors are infused into the portal vein to
promote restoration of insulin function by means of islet
engraftment
in the liver [10]. The 5-year insulin independent rate is 50%
[10].
On US, a normal pancreatic graft should have arterial flow
characterized by relatively sharp systolic upstroke and continuous
antegrade diastolic flow (the resistive index is typically between
0.5-0.7) consistent with low intragraft vascular resistance [11].
Pancreatic graft complications:
In combined pancreas and renal transplants, pancreatic graft survival is inferior to renal graft survival. This is most likely related to a higher incidence of complications such as rejection, ischemia secondary to thrombosis, and pancreatitis.
Acute Pancreatic Rejection:
Up to 60% of pancreatic transplants develop graft rejection.
Pancreatic rejection
occurs synchonously with renal transplant rejection in about 50%
of
cases, while isolated
pancreatic rejection is identified in 15-25% of cases. Hyperacute
rejection is a rare occurence that develops immediately after
transplantation in response to the presence of preformed
circulating
cytotoxic antibodies that cause thrombosis and immediate graft
loss
[9]. Acute rejection is an autoimmune vasculitis that usually
develops
1-3 months after transplantation [9]. Early detection is essential
in
order to institute antirejection treatment and avert graft failure
[9].
Clinical detection of pancreatic
transplant failure is difficult because physical and laboratory
signs cannot accurately diagnose early pancreatic
dysfunction. Serum amylse and lipase are not sensitive or specific
markers of transplant rejection [11]. The sensitivity of serum
amylase is only about 50% for detecting rejection and both amylase
and lipase may be elevated in rejection or pancreatitis [11].
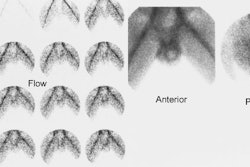
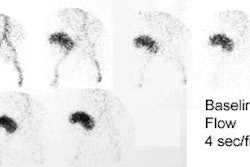
Tc-HMPAO extraction is proportional to blood flow, and may have a role in evaluation of graft function. Lack of Tc-HMPAO accumulation in the transplant indicates either vascular thrombosis or graft pancreatitis.
On ultrasonography, the most common finding in acute rejection is pancreatic enlargement (sensitivity 58%, specificity 100%). Other findings include loss of marginal definition, focal or diffuse areas of decreased echogenicity (heterogenenous parenchymal echotexture), and the presence of peripancreatic fluid collections, however, these findings are very non-specific as they can also be seen in cases of pancreatitis and ischemia. An increase in the resistive index (greater than 0.7) has also been reported to correlate with rejection (sensitivity 20%, specificity 73%). However, because the pancreas lacks a capsule, an edematous graft may not possess adequate intraparenchymal pressure to produce a reliable measurement of vascular resistance [5] and other authors state that resistive indices are not useful for diagnosing acute rejection [9].
Unfortunately, gray scale and doppler sonography are inferior to
sonographically guided
percutaneous biopsy for confirmation of rejection. This is because
the pancreas lacks a capcule and despite edema associated with
rejection, the rejecting pancreas can still demonstrate normal
flow and vascular resistance [11]. Complication rates
from the procedure are low (under 2%) and a
diagnosis can be obtained in 88-95% of cases. [1].
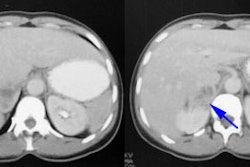
Contrast enhaced CT may show decreased or heterogenenous
enhancement
of the trasplant due to small vessel occlusion [9].
MRI can also be used to assess for pancreatic rejection [2].
Chronic pancreatic rejection:
Chronic rejection occurs in 4-10% of patients and manifests as an
insidious loss of exocrine and then endocrine function [9]. Chronic
rejection results in marked pancreatic atrophy [9].Graft pancreatitis:
In the early post-operative period (less than 4 weeks after transplant), mild self-limited pancreatitis is common (up to 35% of patients) [9]. This usually occurs because of reperfusion injury and risk factors include donor age, cold ischemic preservation time (prolonged warm ischemic time) , and handling of the organ during surgery [9].Vascular Complications:
Graft thrombosis, including both venous and arterial, is second only to rejection as a cause of graft failure after pancreatic transplantation (5-14% of transplants). Venous thrombosis can lead to hemorrhagic pancreatitis, organ necrosis, infection, and thrombus propagation.
1. Venous thrombosis: Venous thrombosis is estimated to occur in 5% of pancreatic transplants and is more common than arterial thrombosis [9]. Acute thrombosis typically occurs in the first 6 weeks after transplant and is more common in enteric drainage than in bladder drainage [9]. Predisposing factors include underlying coagulopathy, long preservation time of the transplant, poor quality native donor vessels, left sided graft placement, and use of a venous extension graft. Venous thrombosis can be difficult to diagnose. Clinical signs and symptoms are non-specific amd include unexplained hyperglycemia, graft tenderness, and hematuria or decreased urinary amylase levels when bladder drainage has been performed [10]. Abnormally high-resistance arterial flow and flow reversal in diastole can be seen [9]. Reversal of diastolic flow in pancreatic transplant arteries is highly specific for detection of graft venous thrombosis during the first 12 days after transplantation. A resistive index greater than or equal to 1.00 and absence of venous flow help to confirm the diagnosis. [3]
2. Vascular stenoses and kinks: All anatomoses can develop stenosis [6].
Anastomotic exocrine leak:
Anastomotic leaks occur in 2-10% of patients following enteric drainage [9]. Early leaks may be attributable to surgical factors, while late leaks are thought to result from infection, rejection, pancreatitis, or duodenitis [9]. Leaks in enteric-drained tranmsplants occur at the duodenal-jejunal anastomosis and can lead to peritonitis, abscess formation, and graft loss [9]. Treatment is immediate surgical revision [9]. For bladder-drained transplants the leak is isially less serious and may be treated with bladder catheterization if there is no evidence of peritonitis [9].Urine Leak following Pancreatic Transplant:
Urine leak from the duodenal segment which occurs in 9 to 14% of
cases. Such leaks may
occur at any time after the procedure- even as late as 5 years
after
tranplantation.
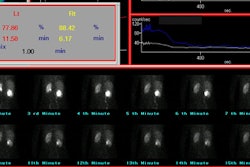
Technetium VCUG has been shown to be more sensitive than
conventional
VCUG in the
detection of such leaks due to superior detection of leaks on the
post-void images [4].
Reversed diastolic transplant flow on US:
Reversed diastolic flow represents the extreme end point of high
vascular resistance [8]. The finding is rare, but often portends a
poor
outcome if not addressed immediately [8]. Etiologies of reversed
diastolic flow include acute rejection (up to 50% of cases and
commonly
associated with transplant failure - especially in long standing
allografts [8]), renal vein thrombosis (rare, but high potential
for
graft loss), acute tubular necrosis, extrarenal compression
(peritransplant hematoma), vascular kinking, and
glomerulosclerosis [8].
Post-transplant lymphproliferative disorder:
PTLD develops in up to 6% of pancreatic transplant patients
typically with widespread involvement of lymph nodes and the liver
(39-40% of cases) [9].
REFERENCES:
(1) AJR 1996; 166:803-807
(2) Radiology 1999; Krebs TL, et al. Acute pancreatic transplant rejection: evaluation with dynamic contrast-enhanced MR imaging compared with histopathologic analysis. 210: 437-442
(3) AJR 1997; Diagnosis of venous thrombosis by duplex sonography. 169: 1269-1273
(4) AJR 1995; Aug. p.349-354
(5) Radiographics 2003; Nikolaidis P, et al. ROle of sonography in pancreatic transplantation. 23: 939-949
(6) AJR 2005; Hagspiel KD, et al. Contrast-enhanced MR angiography after pancreas transplantation: normal appearance and vascular complications. 184: 465-473
(7) AJR 2006; Lall CG, et al. Bowel complications seen on CT
after
pancreas transplant with enteric drainage. 187: 1288-1295
(8) AJR 2008; Lockhart ME, et al. Reversed diastolic flow in the
renal transplant: perioperative implications versus transplants
older
than 1 month. 190: 650-655
(9) Radiographics 2012; Vandermeer FQ, et al. Imaging of
whole-organ
pancreas transplants. 32: 411-435
(10) Radiographics 2013; Low G, et al. Imaging of vascular
complications and
their consequences following transplantation in the abdomen. 33:
633-652
(11) Radiology 2015; Tolat PP, et al. Pancreas transplant
imaging: how I do it. 275: 14-27