General Nuclear Medicine
Generators:
Molybdenum 99 is the parent isotope of Tc-99m. Mo-99 has a
half-life of 66 hours (gamma emission 740 keV).
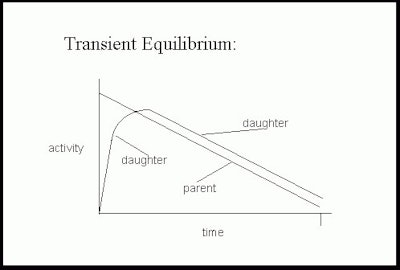
Molybdenum-Technetium generators provide an example of "transient
equilibrium" which is seen when the half-life of the parent
nuclide is moderately longer than that of the daughter. If the
generator is left untouched an equilibrium occurs between the rate
of Mo-99 decay (and therefore the rate of Tc-99m accumulation) and
the rate of Tc-99m decay. It takes about 4 daughter half-lives to
reach equilibrium. When the equilibrium is reached, the amount of
Tc-99m available is approximately equal to the amount of Mo-99
present and the entire system decays with an effective half-life
of Mo-99. (The Tc-99m radioactivity decays with an apparent
half-life of the parent radionuclide Mo-99). The actual amount of
Tc-99m available is slightly less than the Mo-99 activity due to
about 10% of Mo-99m decaying directly to Tc-99.
The column within the generator is made of alumina (Al2O3) and Mo-99 is adherent to this column. When the Mo-99 in the generator (in the form of molybdate - 99MoO4-2) decays it forms Tc-99m-pertechnetate (TcO4-). Because it has only a single charge, saline elutes Tc-99m in the form of sodium pertechnetate (NaTc-99m04) from the column (it is less tightly bound), while Mo-99 remains attached to it. Following elution the Tc-99m activity quickly reaccumulates, reaching a maximum in about 23 hours. About 50% of this peak activity is reached within 8 hours, so it is feasible to elute the generator every few hours if necessary.
Radiopharmaceutical Quality Control:
QC of the radiopharmaceutical can be separated into categories of sterility, chemical purity, radionuclide purity, and radiochemical purity.
Sterility
The generator and product kits are tested for sterility and pyrogens by the manufacturer during production. Pyrogens are bacterial endotoxins which produce a febrile response. The limulus test is used to detect the presence of pyrogens. The test uses an agent derived from the blood of the horseshoe crab (limulus polyphemus). The limulus extract forms a gel when mixed with endotoxins.
Chemical Purity
Aluminum (Al+3) is a chemical contaminant which can be found in the Technetium eluate, but this is rarely a problem with modern day generators. Aluminum can interfere with some labeling reactions. The United States Pharmacopeia (USP) limits the amount of aluminum which can be detected in the eluate to less than 10 ug/ml.
Radionuclide Purity
Molybdenum is the most important radionuclide contaminant. Each time a generator is eluted it must be evaluated for Mo-99 breakthrough. When given intravenously molybdate is phagocytized by the reticuloendothelial system. Its long half life and beta emissions result in a very high radiation dose even from only a small amount of activity. Current regulations limit Mo-99 activity to 0.15uCi/mCi of Tc-99m at the time of administration (0.15kBq/1MBq). Mo-99 can be produced by fission of U-235 in a reactor, or by irradiation of Mo-98 with neutrons. Mo-99 produced in fission reactions is essentially carrier free. However, other radionuclide contaminants such as I-131, Ru-103, Sr-89, and Sr-90 may be detected. The USP sets limits for these contaminants as well. Less common contaminants which are rarely of clinical relevance include Nb-92, Nb-95, and Zr-95. In the Mo-99 produced by neutron irradiation, the most common impurities are Cs-134, Co-60, Rb-86, and Sb-124.
Radiochemical Purity
Radiochemical impurities are Tc-99m compounds other than the desired radiopharmaceutical. There is always some unlabeled Tc-pertechnetate, as well as insoluble technetium colloids. Reduced technetium can be hydrolyzed to Tc02 or can complex with tin colloids. These stannous or technetium colloids localize to the reticuloendothelial system.
Geiger Counter:
A Geiger-Muller counter utilizes a gas filled tube as the detector and provides a high electron amplification factor and, as a result, high sensitivity [9]. In a Geiger-Muller detector a high electric potential is applied across the gas so that when an incident photon interacts with the gas an ionization cascade is produced to record the event. A quenching gas in the tube quickly restores the system to its baseline. The unit is typically calibrated in counts per minute as opposed to exposure rate (mR/h) (if calibrated for exposure rate this will only apply to the radionuclide used for the calibration procedure) [9]. Although Geiger counters can record individual ionization events, they have very limited count rates. The amplitude of the signals pulses in independent of the energy of the incoming radiation and therefore they cannot measure the energy of the incident radiation or identify radionuclides [9]. They are best suited for low-level surveys and checking for radioactive contamination [9].
Cutie-pie detector:
A cutie-pie detector is a low-sensitivity ionization chamber that is typically calibrated for exposure rate (mR/h) and is used where high-fluxes of gamma rays are encountered [9]. Unlike a Geiger counter, it has a relatively low potential between the anode and cathode, however, the electron signal depends on the energy of the detected x- or gamma-rays [9].
Well Counter:
A well counter is used for high-sensitivity counting of radioactive specimens such as blood or urine samples or contamination survey wipes [9]. The device consists of a cylindric scintillation crystal (most commonly thallium-doped iodide) backed by a photomulitplier tube [9]. They are typically equipped with a multi-channel analyzer for isotope (energy) selective counting [9]. They have high intrinsic and geometric efficiencies and can be used to count activities up to approximately 37 kBq [9].
Dose Calibrator:
A dose calibrator is used to measure samples of radioactivity to the microcurie level. It is a pressurized gas filled ionization chamber with a central well. Dose calibrators cannot discriminate between photon energies and cannot be used to identify radionuclides. Gas filled detectors are not as sensitive as those built with a solid material, because there is a smaller chance of an interaction between an incident photon and the less dense gaseous material.
The four quality control procedures required for the dose calibrator are: constancy, accuracy, linearity, and geometry.
Constancy: Checked at installation and then daily [20]. For constancy, a reference source (57Co, 137Cs, 68Ge, 133Ba) is placed into the calibrator and the activity reading is recorded [9]. The values obtained for constancy must be within 10% of the theoretical activity of the source [9].
Linearity: Checked at installation and quarterly thereafter [20]. The test can be performed by measuring a decaying Tc-99m sample with assays of activity every 12 hours over 3 days or by using lead attenuators of increasing thickness (decay equivalent thickness) [9]. Readings must be within 10% of those predicted.
Accuracy: Checked at installation and annually thereafter [20]. Two separate reference sources are used and measured values must be within 10% of the theoretical activity [9].
Geometry: Checked at installation and following any service [9,20]. Dose readings should be consistent regardless of the volume of the agent.
Gamma Camera Characteristics:
When ionizing radiation interacts with matter the absorbed energy is usually converted to heat. Some substances emit a portion of this excess energy as visible or ultraviolet light and are known as scintillators. The brightness of each flash is proportional to the amount of energy deposited in the crystal, which is proportional to the energy of the incident photon. Thus, both the number and energy of incident photons can be recorded. Scintillation detectors consist of a scintillation crystal and a photomultiplier tube (PMT). Sodium iodide (NaI) is the most commonly used scintillation crystal. It has the highest conversion efficiency- about 13% of the energy deposited in the crystal is emitted as visible light. The performance of NaI crystals is maximized by adding a small amount of ionic thallium- NaI(Tl). In a conventional gamma camera, light from a scintilation is sampled by an array of photomultiplier tubes (tubes closest to the scintillation event receive more light than those that are more distant) which generate an energy signal proportional to the amount of detected light, as well as an x,y coordinate pair estimating the location of the scitillation event [6,18]. The event is recorded if the event falls within the pulse-height analyzer energy window [6]. Newer gamma cameras have grooves machined into the back surface of the NaI(Tl) crystal which limit the spread of light, resulting in improved intrinsic spatial resolution [6]. Unfortunately, gamma cameras are inefficient [10]- conventional Auger cameras detect less than 0.01% of incoming photons [21]. Every time a 140 keV gamma ray scintillates in a NaI(Tl) crystal, it produces 5,600 light photons, which are converted to 700 photoelectrons, which must then be amplified in a photomultiplier tube to produce an electronic signal suitable for information processing [10]. PMT's have very large electronic gains (106) with relatively little noise, however, there conversion efficiency is low (about 20%) and this leads to a significant loss of signal [6]. It is also difficult to maintain the long-term stability of PMT's because they are susceptible to environmental influences such as teperature, humidity, and magnetic fields [6]. They are also bulky and expensive [6]. The greatest factor contributing to Anger camera dead time arises from the need to compute the location of the scintillation within a large NaI(Tl) crystal from the pattern of currents flowing through many photomultiplier tubes [10]. The Anger camera energy resolution is about 9-10%- this means a greater fraction of scattered events being recorded [10].
The photon energy resolution of the NaI crystal is limited due to
the physical characteristics of the detector and the
photomultiplier tubes [13]. The spatial resolution is reduced due
to the geometeric characteristics of the collimator and limited
number of photomultiplier tubes [13]. The sensitivity of the
camera system is very low sine the low-energy high-resolution
collimator attenuates the majority (>99.9%) of the incident
photons [13]. These limitations lead to a relatively large amount
of administered radiation doses, and/or prologed imaging times
[13].
Solid state semiconductor detectors such as germanium or lithium drifted silicon show a transient change in electrical resistance when irradiated. The resultant signal produced by the semiconductor is proportional to the amount of energy absorbed. The number and energy of incident photons can therefore be recorded. The energy resolution of semiconductor detectors is superior to that of a scintillation detector- about 5.7% for a Cadmiun-Zinc-Telluride (CZT) detector [10]. Improved energy resolution results in fewer recorded scattered events [10] (the scatter component of the measured data is decreased by 30% [21]) and can also permit simultaneous acquisitions of different isotopes such as Tl-201 and Tc-99m [13]. CZT detectors have a five-fold increased count rate compared to NaI(Tl) cameras and can process >10 million photons/sec/mm2 [13,14]. As a result, exams can be acquired using lower doses and in less time [14]. Overall, compared to NaI cameras, CZT detectors improve energy, spatial, and contrast resolution through direct conversion of a photon to an electrical pulse without the use of photomultiplier tubes [15,17]. In order to function properly, CZT detectors thickness needs to be kept to a minimum (approximately 5 mm) [18]. Due to the necessary thinness of the CZT detector, its intrinsic efficiency for detecting 140 keV gamma rays is approximately equivalent to that of a conventional sodium iodine detector (88-90%) [18]. Presently, solid state cameras are expensive, difficult to fabricate, and may some require supercooling for efficient performance (although CZT detectors can operate at room temperature [13]).
Solid-state photon converters are more rugged, compact (photomultiplier tubes are not required [21]), and largely immune to environmental influences [6]. They also operate at a lower voltage than PMT's and have a much higher quantum conversion efficiency [6]. Silicon photomultipliers are one such solid-state converter, but they have not yet been integrated with gamma cameras [6].
Spatial resolution
Spatial resolution denotes the ability of an instrument to reproduce fine detail. The resolution is usually expressed as the "full-width-at-half-maximum" amplitude (FWHM). If two points are separated by a distal of FWHM (or more) they should appear separate in the image. If the points are separated by less than the FWHM distance, then the two points will be visualized as only a single point. The larger the FWHM value, the greater the image blur. Smaller FWHM values therefore indicate better detector resolution. Most Anger scintillation cameras have spatial resolutions of 6 to 8 mm at the surface of a high-resolution, parallel hole collimator. Collimators generally improve resolution by excluding scatter.
Sensitivity
Sensitivity (or efficiency) denotes the fraction of incident photons that an imaging system actually records. High energy photons are more penetrating and so are less likely to interact than those with lower energy. Modern gamma cameras perform best when imaging photons in the 100 to 200 keV range. Crystals of greater thickness intercept a higher fraction of photons than do thinner crystals. Unfortunately, thick crystals degrade resolution because light diffuses over a longer path before it reaches the PM tube. Anger cameras locate scintillations by comparing pulses from all PM tubes to determine the position of greatest signal. The best trade off of sensitivity and resolution for imaging Technetium or Thallium is a 1/4 inch (0.6 cm) thick crystal. This thickness, however, is inefficient for the higher gamma emissions of Gallium, Indium, and Iodine. As a compromise, a 3/8 inch (0.95 cm) thick crystal is typically employed.
Temporal resolution
The typical scintillation detector system's temporal resolution is about 1.5 to 3 microseconds. Deadtime (the time during signal processing which other incoming signals cannot be recorded) losses are about 10 to 30% with a maximum count rate of about 300,000 counts per second. Multi-crystal cameras can record up to 50,000 counts per second in each crystal/PM tube resulting in count rates of 600,000 to 1 million counts per second. The thicker crystal used in multi-crystal detectors (1 cm), however, limits spatial resolution.
Collimators:
Collimators play a crucial role in defining a systems extrinsic imaging characteristics. Collimators will reject photons that are not within a small angular range [11]. Collimators therefore exhibit low geometric efficiencies (defined as the percentage of detected to emitted photons) of the order of approximately 0.01% [11]. The energy rating of a collimator indicates the maximum energy of photons that can be efficiently handled by the collimator. This is usually defined as the energy at which less than 5% of the off-axis photons pass through the collimator. Low energy collimators are designed for a maximum energy of 140 to 200 keV, while medium energy collimators are effective up to 300-400 keV. The energy rating of the collimator also dictates septal thickness. Although tungsten absorbs photons more efficiently, most collimators are made of lead due to its lower cost.
Resolution and Sensitivity
Resolution and sensitivity of a collimator are inversely
related. The best spatial resolution is achieved with a
collimators with long holes of a small diameter because the
angle of acceptance is smaller and more scatter is rejected. The
sensitivity of this high-resolution collimator, however, is
lower because fewer photons reach the crystal. Sensitivity, or
efficiency, refers to the fraction of emitted photons which
actually pass through the collimator and reach the detector. The
sensitivity increases as the square of the hole size, and
decreases as the square of the hole length. When spatial
resolution is not critical and photon flux or acquisition time
is limited, a high sensitivity collimator can be used. The
all-purpose collimator is a compromise between sensitivity and
resolution.
When using any collimator, spatial resolution decreases with increasing distance from the patient [16]. As a rule of thumb, resolution falls about 1 mm for each additional centimeter that a patient is positioned from the face of a parallel-hole collimator.
Thus, collimator resolution improves as:
? The diameter of the collimator holes decreases
? The effective length of the collimator holes increases
? The object to collimator distance decreases
Types of Collimators:
Parallel hole
Parallel hole collimators introduce no image distortion and
provide a constant field of view. Resolution is best when the
object is placed against the collimator, but decreases with
distance from the camera face [18]. The magnitude of the loss of
resolution is directly proportional to the width of the
collimator hole and inversely proportional to the hole length
[18]. Septal penetration by photons is another cause of
decreased resolution for a parallel hole collimator and is
related to the thiclness of the septa [18]. The sensitivity of a
parallel hole collimator is independent of distance from the
collimator.
Low-energy high resolution collimators have a resolution of about 7mm at 10 cm from the collimator surface [12]. General purpose collimators typically have 2x's higher sensitivity compared to high resolution collimators, but a reduced collimator spatial resolution of 9 mm [12]. High sensitivity collimators have 4x's higher sensitivity than high resolution collimators, but their spatial resolution is only about 13mm [12]. Switching from a "high resolution" to a "general purpose" collimator allows one to reduce the patient dose by 50% to achieve the same number of counts, with only a slight decline in system resolution [12].
Converging and Diverging
Converging and diverging collimators are named by viewing the holes from the detectors perspective. Converging collimators project an object onto a larger portion of the crystal and thus magnify the image. A converging collimator may actually allow detection of structures smaller than the cameras intrinsic resolution (increase system resolution). In practice, magnification is limited to about 15%, however, converging collimators do introduce image distortion as magnification varies based upon the depth of the object. The collimators sensitivity (efficiency) increases with distance from the collimator, but this will decrease resolution.
A diverging collimator minifies the image and degrades both sensitivity and resolution, which will worsen with increasing distance from the collimator. It is useful only when it is necessary to image a structure larger than the size of the detector.
Pinhole
A pinhole collimator can also be used for image magnification. Maximum magnification results when the pinhole is closest to the object and decreases as the pinhole is moved further away. Because magnification is determined by distance from the aperture, a thick object contains areas that will be imaged at different magnifications. This differential magnification produces image distortion that is greatest at high magnification. Pinhole images also increase the spatial resolution of the camera system. Resolution is related to the size of the aperture, however, small apertures which produce the best resolution limit the number of accepted counts. Resolution is also improved by increasing the length of the aperture. Pinhole images are best for magnifying small, thin objects. Electronic magnification is easier to perform, but it does not increase system resolution.
Camera Quality Control:
The parameters most commonly evaluated for routine gamma-camera include uniformity, spatial resolution, spatial linearity, and energy resolution and peaking [9]. Each camera should be peaked daily and before switching to a new radionuclide to ensure that the energy window is correct. Uniformity verifies that there are a proportional number of counts recorded at every location on the scanner [19]. Uniformity should also be checked on a daily basis using a high count flood. This flood can be extrinsic (with the collimator on) or intrinsic (with the collimator off) [9]. For an intrinsic flood, the collimator is removed and a point source is placed at least 5 times the detector diameter away [9]. From this distance an essentially uniform photon flux will strike the detector [9]. A point source cannot be used to perform an extrinsic flood because the collimator will exclude off-axis radiation. Extrinsic floods must be performed with either a disc source or a water filled phantom. Disc sources are made from Cobalt-57 which has a gamma energy of 122 keV and a half-life of 272 days and are manufactured to present a very uniform field. The advantage of an extrinsic flood is that it can detect the presence of a damaged collimator. A total of 10-15 million counts is acquired and uniformity is quantitated for the integral and differential uniformities [9]. Uncorrected images will generally demonstrate a slight heterogeneity due to PM tube imbalances or non-uniformity of the crystal itself. A variation in uniformity of up to 5% can be tolerated for planar imaging [9] without substantial loss of image detail. For SPECT imaging, however, variation in uniformity across the detector should be less than 1%. Correction circuitry is used to make the image uniform.
Spatial linearity (the ability of the system to accurately render
straight lines) uses a bar phantom to verify that counts received
by the detector are recorded in the correct location [19]. Camera
resolution and spatial linearity should be checked weekly at a
minimum using a four quadrant bar phantom [9]. The lead bars
in at least two of the quadrants (3 and 4-mm bars) should be
resolvable (and probably a portion of the quadrant with the 2.5 mm
bars) [9]. All bars should appear straight [9].If the linearity
phantom demonstrates nonlinearities- such as jagged lines or a
wavy pattern, the system should not be used until serive can
correct the problem [19].
SPECT Imaging:
SPECT is superior to planar imaging with regard to disease localization. SPECT imaging improves object contrast (i.: contrast resolution or the target to background ratio) by removing overlying tissues. Spatial resolution is degraded (Spatial resolution is about 1.0 cm which is similar to the LV wall thickeness [8]) and the loss of spatial resolution is depth-dependent [8]. With a circular orbit, the detector will at times be far from the patient which results in a loss of spatial resolution. Elliptical (or contouring) orbits will keep the detector closer to the patient and therefore should not suffer from this same loss of spatial resolution. Unfortunately, particularly with cardiac SPECT, elliptical orbits actually result in a greater likelihood of artifact. Although the detector is closer to the heart, there is actually a greater variation in detector distance from the heart than with a circular orbit (i.: with the use of a circular orbit, the detector to cardiac distance, although greater, is more constant throughout the arc of the detector). The greater variation in spatial resolution associated with elliptical orbits can produce perfusion defects in the inferior and anteroapical regions. This artifact is more apparent in thin patients and is not observed if a 360 degree orbit is used. Another problem with SPECT cardiac imaging is that portions of the LV that are closer to the detector (such as the lateral wall) can appear brighter (i.e.-thicker) than other segments [8]. Due to partial volume effects, hypertrophied segments will also show more brightly [8]. Due to LV hypertrophy, hypertensive patients may have a decrease in the normal lateral-to-septal ratio [8].
SPECT imaging is also degraded by photon scatter [7]. In typical SPECT imaging, as much as 15% of all photons collected in a Tc-99m acquisition have been scattered [7].
Field Uniformity:
Detector field uniformity is the most crucial parameter of SPECT performance. Random fluctuations in flood field sensitivity (field non-uniformities) must be kept below 1%. To ensure flood uniformity and a percent relative standard deviation of 1% with 10,000 counts per pixel requires a 30 million count flood for a 64 x 64 matrix (120 million counts would be required for a 128 x 128 matrix). Typically these high count floods are performed weekly, with 3 to 5 million count floods daily. Floods should be obtained with the collimators on. Field non-uniformity artifacts have a bulls-eye appearance with alternating concentric rings of high and low intensity. Spatial non-linearity does not generally lead to reconstruction artifacts, but does contribute to intra-slice resolution.
Center of Rotation:
The detectors of a SPECT camera rotate around a central axis and single point in space. The computer makes assumptions about the location of this axis during image reconstruction. The center of rotation corrects for the difference between the center of the computer matirx and the projection of the cameras face. If the COR is calibrated correctly, a point source placed in the center of the cameras orbit during a SPECT acquisition will appear as a point in the center of the computer matrix. If the actual axis does not correspond to the assumed axis, artifacts will be created during image reconstruction (the point will appear blurred or as a ring artifact). Most departments check the center of rotation weekly. Less frequent checks are possible with multi-head detector systems fixed in a rotating gantry.
The COR acquisition consists of a point source placed off-center in the field of view. COR should be calculated to a precision of at least one-tenth of a pixel. A shift of 1/2 pixel in the center of rotation can result in significant image degradation. An alignment error in the axis of rotation will result in a point being reconstructed as a ring, thus COR artifacts typically have a "comet tail" or ring-like appearance. Since the artifact is equipment related and constant, it will be present on both stress and rest cardiac images.
Detector Alignment:
The camera head should be leveled prior to each acquisition. An non-lev head will create an artifact similar to COR misalignment. To evaluate detector alignment, the point source used for COR determination should be viewed in a cine mode. The point sources should move back and forth in a straight line. If it oscillates up and down, the camera head is not level, or the head support apparatus is not truly vertical.
Patient motion:
Patient motion is a considerable problem in tomographic imaging which relies on a accurate center of rotation.
Factors which affect the final result of motion include:
1. Amount of movement: Less than 3mm is not usually visually detectable. Movement less than 6.5mm is detectable, but is not usually clinically significant. Movement of 13mm or greater (2 pixels) frequently produces quantitative abnormalities.
2. Type of movement: Axial movement (up and down) is usually more significant than lateral movement.
3. Time of movement: Movement at the beginning or end of a study is less likely to result in image artifacts, whereas movement occurring at midaquisition has the worst effect.
4. Multiheaded camera: Dual detector cameras are more vulnerable to motion than are single detector cameras because twice the number of projections are affected [1]. Also, the second detector begins it acquisition at the point just beyond where the first detector completes its movement. Any movement which occurred during the exam will appear at this point in the rotating planar images. On the other hand, because acquisitions on single headed cameras take longer, the chance that the patient will move during the exam is greater [1].
Patient motion can be evaluated in a number of ways:
Sinogram
Corresponding lines (horizontal profiles) from each planar view (i.: equivalent projections as used to reconstruct a given tomographic plane) are arranged consecutively in a two-dimensional array.
Cinematic display
Cinematic display of acquisition images (can also assess soft tissue attenuation).
Summed Projection Image
The multiple projection images are added together to form a composite projection image. The heart should form a horizontal line unless motion has occurred.
Tomographic Reconstruction:
As a rule of thumb, in order for SPECT to obtain the same statistical accuracy as conventional planar imaging, about 5 times as many counts are required. However, it is better to have a somewhat count poor, but otherwise sharp image, than a high count blurry one. The use of multiple detectors provides sufficient sensitivity to allow the use of the highest resolution collimators available, yet still achieve adequate information density. [2]
Attenuation Correction:
Nuclear medicine images are degraded by various photon interactions. Photon attenuation significantly degrades the quantitative accuracy of SPECT images by introducing image artifacts and distortions. The single most important factor degrading SPECT image resolution is the distance from the camera to the patient. This occurs due to a change in resolution with object distance from the detector- i.e.: the further an object is from the detector, the worse the resolution [5]. The second most important factor is scatter due to self-absorption within the patient (Compton scatter [CS]). CS refers to photons that are emitted from the patient and interact with other atoms- not enough to be absorbed, but enough to change direction and lose energy [5]. When these scattered photons reach the detector they are recorded as if they had traveled along a straight path- when in fact they are incorrectly located which reduces image contrast [5].
Tc-99m has a half-value layer of approximately 4 cm in soft tissue. Regions near the center of the body or brain are at least 2 HVL or more from the gamma camera than more superficial structures. This extra depth will result in significant variation in emissions reaching the camera due to self absorption. These self attenuation losses must be corrected for electronically during reconstruction as a true attenuation correction is not presently available for SPECT imaging. The two methods most commonly used for attenuation correction are the Sorenson pre-processin method, and the Chang post-processing method. These methods do not work well in the thorax since the heart is surrounded by tissue of varying density (lung, chest wall, and bone) which have different attenuation coefficients. In nuclear cardiology exams, attenuation correction has been shown to improve specificity and normalcy rates, without decreasing sensitivity [5]. True attenuation correction requires the use of a transmission scan to determine appropriate corrections. The transmission scan can be accomplished by obtaining a 10 minute SPECT image from a source opposite the patient. The emission patient images may then be corrected appropriately. A combined transmission-emission study can be performed using a 3 headed camera, utilizing 2-heads to collect emission data, and one to collect transmission information. SPECT cameras with built in CT scanners are available. CT provides high quality attenuation maps that are superior to those acquired with radionuclide transmission scanning [3]. However, misalignment between the transmission and emission studies can be a major source of artifacts [3] and misalignment can be found in up to 42% of studies [5].
Filtered back-projection:
Filtered back-projection is used for image reconstruction.
Back-projection alone (without filtering) results in undesirable
image smoothing and the presence of star-like artifacts. The
degree to which back-projection artifacts can be removed must be
balanced by the degree to which image noise can be tolerated.
Frequency refers to the change in number of counts from pixel to
pixel. True image signal falls off rapidly with increasing
frequency, while the noise content remains constant. Background
(noise) is considered to be high frequency because there is marked
variability in the number of counts from pixel to pixel. Image
sharpness (edge detection, small objects, and fine detail) are
also high frequency, while the target (a large object) is low
frequency. FBP basically suppresses high-frequency image data,
thereby deceasing noise [18]. However, in doing so the technique
results in image blurring that can obscure subtle findings [18].
The smoother the filter (i.e.- the lower the critical frequency
and the higher the order) the greater the degree of image blurring
[18].
RAMP filters (high pass filters) boost high frequencies in order to sharpen the spatial details (edges) of the image and to minimize the star artifact [1]. Unfortunately, this also increases the noise because the filter linearly enhances higher frequencies. Thus, although RAMP filters produce the highest resolution possible in a reconstruction, the images are often uninterpretable due to the propagation of noise associated with low count statistics. To limit this effect (i.: to decrease the noise) a second roll-off or low pass (or "smoothing") filter is applied. Low pass filters let low frequencies "pass" and progressively attenuate higher frequencies. A low pass frequency filter is therefore employed to reduce statistical noise and smooth the image. Low pass filters are typically applied to projection images before reconstruction to reduce reduce noise early in the processing chain [1]. Low pass filters increase the signal to noise ratio, but at the expense of image contrast, edge definition, and resolution. Common low pass filters include: Butterworth, Hanning, Shepp-Logan, and Parzen. Butterworth filters are the most commonly used for nuclear medicine procedures [1].
When selecting a processing filter there is always a trade-off between image contrast and image uniformity. The cut-off frequency is the frequency above which all data is removed. The lower the cut-off frequency employed, the smoother the reconstructed image (more uniform) and the greater the loss of contrast and resolution due to the loss of image sharpness contained in the higher frequency data. High count statistics are crucial, as the higher the number of counts in the projected data, the higher the cut-off frequency can be. A filter with a high cut-off produces images with a lot of contrast which can result in a high sensitivity, but low specificity. The filter order refers to the steepness of the slope of the filter curve. High order implies a steep slope which produces a sharper image, but also creates more image distortions.
Filtering prior to back projection is preferable because: 1- It reduces the propagation of noise at an earlier stage in the image formation process and 2- It promotes the implementation of a filter symmetric in 3-dimensions.
Although a larger matrix array (128 x 128) with smaller pixels would theoretically yield better spatial resolution, each pixel would have proportionately fewer counts and the image would be degraded by statistical noise. The percent variation in pixel counts due to chance or statistical effects is approximately 100%/(N)1/2 , where N is the number of counts per pixel. The statistical variability for a SPECT scan is about 5 to 10% for a 64 x 64 matrix. Doubling the array size in order to improve spatial resolution will approximately double the statistical error in pixel count measurements. This increase in statistical noise will decrease contrast resolution and may obscure image detail.
Iterative reconstruction:
Algebraic reconstruction techniques use projection images as
input, but aim at finding the exact mathematical solution to the
problem of activity distribution in the field of view by
considering the value in each pixel of the reconstructed image as
an unknown and each point in a profile as an equation [1]. In
brief, the value of all pixels is initially guessed using filtered
backprojection; then those initial values are slightly altered
several times (iterations) until they converge to a final result
consistent with the available count profiles [1]. Iterative
reconstruction is intrinsically slower than filtered
backprojection, but it has the clear advantage of reducing
reconstruction artifacts- such as those caused by hepatic or
extracardiac activity on myocardial perfusion imaging [1].
OSEM (ordered subset expectation maximization) iterative
reconstruction (IR) has become the mthod of choice for IR
processing- whereby data are grouped into subsets, allowing more
rapid and accurate data convergence [18]. OSEM images are of
higher quality with improved image contrast than thosed processed
using FBP [18].
Object Size Correction (Finite resolution effects):
Tomographic resolution for a single headed camera is roughly
16-18 mm for thallium, and 13 mm for technetium. For objects
smaller than 2 resolution elements of the detector, the counts
recovered from the reconstructed tomograms are greatly dependent
upon the objects size (the maximal counts will be proportional to
the thickness of the object). In other words, because the
myocardial thickness (10-20 mm) is less than two resolution
elements of the detector, if the activity in the septum and
lateral walls was identical, but the septum was twice as thick as
the lateral wall, the examination would demonstrate a perfusion
defect in the lateral wall. A non-compliant wall may also appear
hotter.
Critical Organs:
EXAM/AGENT
CRITICAL ORGAN
Tc-Pertechnetate
Stomach
Tc-MDP
Urinary Bladder
Tc-DTPA/MAG3
Urinary Bladder
Tc-DMSA
Kidney
Tc-IDA
Gallbladder wall
Tc-SC
Liver
Tc-MAA
Lung
Tc-Sestamibi
Proximal Colon
Tc-DTPA
Aerosol
Urinary Bladder
Tc-RBC's
Heart
Thallium
Renal Cortex
Gallium
Distal Colon
In-111/Tc-99m HMPAO
WBC's
Spleen
Xe-133
Trachea/Airways
I-131/I-123
MIBG
Adrenal Medulla
F-18
FDG
Urinary Bladder
N-13
Ammonia
Urinary Bladder
REFERENCES:
(1) J Nucl Med 2001; Germano G. Technical aspects of myocardial SPECT imaging. 42: 1499-1507
(2) J Nucl Med 1993; Juni JE. Doing well under pressure: dedicated SPECT cameras come of age. 34: 1789-92
(3) J Nucl Med 2004; Fricke H, et al. A method to remove artifacts in attenuation-corrected myocardial perfusion SPECT introduced by misalignment between emission scan and CT-derived attenuation maps. 45: 1619-1625
(4) J Nucl Cardiol 2006; DiFilippo FP, et al. Collimator integrity. 13: 889-891
(5) J Nucl Cardiol 2007; Garcia EV. SPECT attenuation correction: an essential tool to realize nuclear cardiology's manifest destiny. 14: 16-24
(6) J Nucl Med 2007; Madsen MT. Recent advances in SPECT imaging. 48: 661-673
(7) Nucl Cardiol 2007; Singh B, et al. Attenuation artifact, attenuation correction, and the future of myocardial perfusion SPECT. 14: 153-164
(8) J Nucl Cardiol 2007; Jaber WA, et al. Left ventricular hypertrophy and SPECT myocardial perfusion imaging: finding the diamonds in the rough. 14: 398-407
(9) J Nucl Med 2008; Zanzonico P. Routine quality control of clinical nuclear medicine instrumentation: a brief review. 49: 1114-1131
(10) J Nucl Cardiol 2009; Nichols KJ, et al. Prospects for advancing nuclear cardiology by means of new detector designs. 16: 691-696
(11) J Nucl Cardiol 2010; Beanlands RSB, Youssef G. Diagnosis and prognosis of coronary artery disease: PET is superior to SPECT: Pro. 17: 683-695
(12) J Nucl Cardiol 2010; Cerqueira MD, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. 17: 709-718
(13) J Nucl Cardiol 2010; Sharir T, et al. Solid-state SPECT technology: fast and furious. 17: 890-896
(14) J Nucl Cardiol 2011; Lane Duvall W, et al. Reduced isotope
dose with rapid SPECT MPI imaging: initial experience with a CZT
SPECT camera. 17: 1009-1014
(15) J Nucl Cardiol 2011; Henzlova MJ, Duvall WL. The future of
SPECT MPI: time and dose reduction. 18: 580-587
(16) J Nucl Cardiol 2012; Garcia EV. Physical attributes,
limitations, and future potential for PET and SPECT. 19: S19-29
(17) J Nucl Cardiol 2012; Garcia EV. Quantitative nuclear
cardiology: we are almost there! 19: 424-437
(18) J Nucl Cardiol 2012; DePuey EG. Advances in SPECT camera
software and hardware: currently available and new on the horizon.
19: 551-552
(19) J Nucl Cardiol 2013; Case JA, Bateman TM. Taking the perfect
nuclear image: quality control, acquisition, and processing
techniques for cardiac SPECT, PET, and hybrid imaging. 20: 891-907
(20) AJR 2015; Baldwin JA, et al. All you need to know as an
authorized user. 205: 251-258
(21) J Nucl Med 2019; Slomka PJ, et al. Solid-state detector
SPECT myocardial perfusion imaging. 60: 1194-1204