GI Motility Studies
Radionuclide Esophagram:
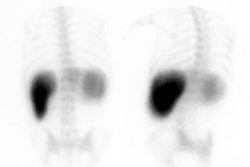
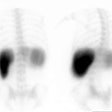
The radiopharmaceutical is 100 to 300 uCi of Tc-SC or Tc-DTPA in 15-30 ml of water [17]. The patient should fast for 3 hours (or over-night) [12]. The patient should initially practice the procedure by swallowing using unlabelled plain water [12]. A high sensitivity collimator should be used to maximize temporal resolution. With the patient erect the liquid is taken into the mouth through a straw as a bolus, and then a single swallow is performed. Other authors suggest that the patient be instructed to swallow multiple times for complete clearance of the tracer from the oral cavity [19]. Rapid sequential images (0.5 sec per frame) are acquired for 30 seconds to 1 minute using a 64 x 64 or 128 x 128 matrix. This is followed by dry swallows at 15 second intervals for up to 10 minutes. Either single anterior or single posterior views of the chest have been used for esophageal transit imaging [17]. Computer analysis can be performed using a region of interest over the entire esophagus.
Alternatively, a single swallow can be performed and computer analysis of regions placed over the upper, mid, and lower thirds of the esophagus can be used to evaluate the progression of the bolus. Activity in the mouth, pharynx, and stomach regions can be monitored to help identify artifacts from delayed swallowing or gastroesophageal reflux.
Total esophageal activity decreases very rapidly in normal
subjects. Transit time can be defined as time (seconds) from
initial swallow to 90% clearance from peak activity [19]. Within 5
to 10 seconds after the first swallow, esophageal activity is
generally no longer visible. Normally, less than 10% of the
administered dose should remain in the lumen after 15 seconds (in
other words, a normal transit time is less than 15 seconds [19]),
but count rates of approximately 10% of the peak activity may be
recorded until the 8th swallow.
Some practitioners measure the decrease in the percentage of
esophageal activity at 10 seconds after peak [17]. This is
referred to as percentage transit and is quantified as the number
of counts at peak minus the number of counts 10 seconds after peak
counts, divided by maximal counts [19]. An esophageal percent
emptying greater than 83% is considered normal [19]. Some authors
comment that less than 5% of peak activity should be detected at
10 minutes, however, other authors suggest that normally more than
82% of the esophagus empties after 10 minutes of serial dry
swallowing [17].
With regional esophageal analysis, there should be a smooth temporal progression in the sequential appearance of the tracer in the proximal, middle, and distal portions of the esophagus. Mean transit time for the entire esophagus is generally 7 +/- 2 seconds. Evaluation of the curves from each region can aid in the differentiation of achalasia, scleroderma, and diffuse esophageal spasm.
Causes of Prolonged transit time:
Achalasia
There is an elevated LES pressure with incomplete sphincter relaxation and aperistalsis of the entire esophagus. The esophageal segmental pattern is typically flat in all segments, with little evidence of bolus propagation. Transit studies may show normal progression of the bolus through the upper and mid esophagus, but there will generally be very prolonged abnormal retention of activity within the distal esophagus.
Scleroderma
These patients typically demonstrate greater than 50% retention of activity in the distal esophagus even after 10 minutes of multiple swallows.
Mechanical obstruction
Stricture, Neoplasm
Diffuse esophageal spasm
There are repetitive high amplitude and non-progressive contractions, but the LES function is normal. The transit study usually demonstrates multiple uncoordinated peaks representing disorganized transit of the bolus through all segments of the esophagus.
Hypertensive lower esophageal sphincter
Non-specific Motor Disorder
Solid or semisolid esophageal emptying studies utilizing radiocolloid labeled corn flakes in milk can be used to assess the results of therapy, particularly in patients with achalasia. In normal individuals there is rapid emptying with less than 5% esophageal retention at one minute after completion of the meal and a further decline to less than 1% by 20 minutes.
Gastroesophageal Reflux:
The barium swallow is an insensitive test for detecting the presence of intermittent reflux, with only 15-20% of affect patients detected on this exam. 24 hour intraesophageal pH monitoring is the best test for the evaluation of reflux.
Gastroesophageal Reflux Scintigraphy in Adults:
Tc-SC 300 to 500 uCi is suspended in 150 ml of orange juice acidified with 150 ml of 0.1 N hydrochloric acid. Acidification increases the sensitivity of the exam by relaxing the LES pressure gradient and delaying gastric emptying. The esophagus can be cleared of activity by giving the patient several ounces of water to drink. The patient is placed in a supine position and images are obtained every 5 seconds for 1 hour. Ten minutes prior to completion of the exam an abdominal binder is applied to increase intraabdominal pressure. The pressure is increased to 20, 40, 60, and 100 mm Hg for each of 30 seconds. Reflux detected at binder pressures less than 100 mm Hg is considered abnormal as only 10% of normals demonstrate reflux at this pressure. Up to 3% reflux is normal, although some patients may have visually obvious reflux at values less than this. There is a 90% correlation with acid pH reflux test. The average sensitivity for reflux detection by this exam is about 70%. The principal shortcoming of this method is its inability to provide continuous long term monitoring. By using a larger dose of Tc-SC (5 mCi) late images can be obtained over the chest to assess for aspiration [9].
There has not been sufficient data reported to evaluate the role of scintigraphy in the detection of pulmonary aspiration in adults.
Gastroesophageal Reflux Scintigraphy in Children:
In the majority of healthy children, GER is a benign self-limited condition which requires no diagnostic investigation. GER, however, occurs in a significant number of infants with failure to thrive, recurrent pneumonia, intermittent wheezing, cough, or apneic events. Children who present with these symptoms should therefore be evaluated for reflux. The presence of GER in children is frequently associated with delayed gastric emptying.
The baby is fed formula (20cc/kg) with 1 mCi (37 MBq) Tc-SC and should be NPO for at least 4 hours prior to the exam. Alternatively, the amount of formula given may be based upon the patients age: less than 3 months- 90cc; 3 to 6 months- 120cc; or over 6 months- 150cc. The volume of liquid is divided into two equal parts- the Tc-SC labeled part is fed first and the second unlabeled part is then given to clear the oropharynx and esophagus. The swallowing portion of the exam is observed to assess for aspiration. Images are then acquired posteriorly over the stomach/esophagus every 10-15 seconds for 1 hour (64 x 64 matrix) with the patient supine. Images should be viewed in cine and summed static modes [19]. Additional spot 5 minute planar images are performed over the chest and abdomen at 0, 1, 2, and 3 hours to assess for aspiration and gastric emptying. There is excellent correlation between scintigraphy and pH probe analysis, and the radiation dose from this exam is less than that from an UGI series. Sensitivity for detecting GER is 75%-100% [12], and specificity 93%.
For data analysis, an ROI is drawn over the region of the esophagus- reflux episodes will appear as sharp spikes [12]. GER occurring within 5 minutes of formula administration is generally ignored as most babies reflux during this time period. Six or more reflux events after this time are considered significant if the child is symptomatic. Episodes of reflux should be characterized as to whether they were confined to the distal esophagus or if they reached the upper esophagus, and how quickly the reflux cleared.
Gastric emptying calculations can also be performed. Most normal infants empty approximately 50% of gastric activity in 1 hour (range 48% to 70%) with a pattern of first order kinetics [1].
Although scintigraphy is capable of detecting small volumes of aspiration, the diagnosis cannot be excluded by a negative exam. The intermittent nature of imaging after the initial 1 hour limits the exams sensitivity as ciliary action alone (in the absence of a cough reflex) will clear Tc-SC from the upper airway with a T1/2 of 1.7 hours. Clearance is even more rapid with an intact cough reflex. Generally, detection of aspiration is higher in patients with more severe pulmonary disease.
Gastric Emptying:
Etiologies for gastroparesis include diabetes (often also
associated with retinopathy, neuropathy, and nephropathy),
neuromuscular causes, autoimmune/connective tissue disease,
certain drugs, postgastric surgical conditions, malignancy, or
idiopathic [17,20]. Delayed gastric emptying (gastroparesis) is
diagnosed when symptoms such as nausea (92% of patients), vomiting
(84%), early satiety (60%), post prandial fullness and abdominal
discomfort/bloating [75%], and pain are associated with objective
evidence of delayed gastric emptying in the absence of obstruction
[16,17]. Rapid gastric emptying is a major factor in dumping
syndrome- characteristically seen after surgery for peptic ulcer
disease with and without vagotomy [16]. However, rapid gastric
emptying has also been demonstrated in some patients with
unexplained nausea, bloating, and fullness [16]. The early
symptoms of dumping occurring in the initial hour after meal
ingestion include diarrhea, abdominal discomfort, nausea,
bloating, and vasomotor symptoms [16]. Some of the symptoms of
dumping can be difficult to distinguish from gastroparesis
symptoms [16]. The late dumping syndrome presents with
diaphoresis, palpatations, weakness, and fainting secondary to
reactive hypoglycemia from exaggerated insulin release [16].
The goal of diagnosing delayed gastric emptying is to identify
patients who will benefit from either prokinetic drug or other
treatment to alleviate their symptoms [17].
The rate of emptying of a meal varies depending on the type of food, the volume of the meal, and its caloric content. High caloric substances such as fat empty more slowly than carbohydrates. The most important factor effecting liquid gastric emptying is the number of kcal contained in the liquid meal. Upright positioning and ambulation have been described to speed gastric emptying. Other factors that are believed to affect gastric emptying include the osmolality, acidity, and chain length of fatty acids in the meal. Differences in emptying may also exist between males and females. The time of day of the exam may also affect the exam with more rapid emptying observed in the morning following an overnight fast. Because of the above factors, it is important that each institution develop its own set of normal standards for the particular meal that is used at that institution. It has also been suggested that normals values with standard deviations need to be developed for both male and female subjects.
Liquid Meals
It has generally been taught that liquids empty in a
monoexponential pattern with no lag phase- thus, the volume of
fluid emptied into the duodenum in a given time is a constant
fraction of the volume remaining in the stomach [12,17]. This is
only true for low calorie content liquids, principally water.
Calorie containing solutions tend to empty linearly if they have
a sufficient calorie content. It has been well established in
the physiology literature that liquid calories empty from the
stomach at approximately 2.0 kcal per minute [2,3]. Therefore,
there is no correct half-emptying time for liquids because the
half-emptying time all depends on the total calories contained
in the liquid given. Once a solution containing more than 25
kcal is given the solution starts to empty a slower rate than
water. The normal half-emptying time for noncaloric liquids such
as water is 10-15 minutes (other authors indicate < 25
minutes) and noncaloric fluids empty in a monoexponential
pattern [19]. Delayed liquid emptying can be seen in 24-33% of
patients with normal solid gastric emptying [19].
Gastric emptying of liquids is a less complex process because no grinding is required. Liquid emptying is controlled by the fundus (as opposed to solid emptying which occurs in the antrum where phasic contractions grind food into small particles (1-2mm) so they can pass through the pylorus) [19]. After ingestion of a meal, the fundus relaxes and accommodates the meal volume [15]. The smooth muscle of the fundus then contracts in a tonic manner producing a pressure gradient between the fundus and the pylorus (this is responsible for liquid emptying) [15]. Under normal conditions liquids will begin to leave the stomach almost immediately after ingestion [12], however, other authors have reported a short lag phase even for water [15].
Solid Meals
Because solid meals are high in caloric content compared to
water, they empty at a constant rate (Zero order kinetics or
linear pattern of emptying). The control of the emptying of
energy containing meals is exerted by relaxation of the fundus
with feedback being provide from receptors stimulated in the
small intestine or from central centers of glucose, amino acid
or fatty acid levels in the brain.
With normal meal ingestion, the proximal gastric fundus relaxes
and increases in volume to accommodate the meal [18]. Impaired
fundic accommodation compromises the ability of the upper
stomach to act as a reservoir and can result in accelerated
transit from the proximal to distal stomach (impaired fundal
accommodation can be associated with dyspeptic symptoms) [18].
This can be visualized as decreased fundal activity on immediate
post meal imaging (however, it is important to be sure that the
patient consumed the entire meal in a normal amount of time as
these can result in apparent decreased fundal activity on
immediate imaging) [18].
The antrum also plays an important part in the emptying of solid meals [15], as foods must be reduced to small particle sizes (less than 1mm) before they can be emptied into the intestine. The antrum accomplishes this by contracting against a closed pylorus, which results in a reduction in particle size so that food may pass through the pylorus [15]. The antrum does not appear to play a significant role in the emptying of liquids because no particles are present.
Lag Phase
The lag phase is the delay before activity appears in the bowel. With solid meals, there is normally an initial "lag phase" during which time little or no gastric emptying is observed. The lag phase represents the time required for the transfer of solid food from the fundus to the antrum and then for the antrum to grind the food into small particles so that it can begin to empty into the duodenum [12]. The antrum reduces the size of the solid particles to less than 1 mm so that it can easily pass through the pylorus [11]. If a radiolabeled solid meal is ingested with a high calorie liquid meal, the lag phase of emptying of the radiolabeled solid meal will be even longer because of the fact that most liquids emptying first before solid emptying and the high calorie liquid meal will take some time to empty before the solid meal begins to empty. The lag phase can be identified as an inflection point along the emptying curve (or the time of peak activity in the antrum which corresponds to maximal filling of the antrum just before the adequately triturated small, suspended solid particles begin to empty at the same uniform rate as liquids [17]).
Gastric Emptying Radiopharmaceuticals
Tc-SC (0.1 to 1 mCi) or In-111 DTPA (0.1 to 0.5 mCi) with water
are the agents used to evaluate liquid gastric emptying. The use
of In-111 DTPA permits a dual tracer liquid-solid emptying
determination [17]. Liquid emptying has been shown to be abnormal
in 5-26% of patients with normal solid emptying [17].
Commonly Used Meals & Technique
- Calorie content highly reproducible with every administration, unlike to the caloric variation with eggs and toast or even milk and cereal.
- Lemon lime flavor which is well tolerated by virtually all subjects.
- No problems with lactose intolerance or egg allergy.
- The osmolarity of the glucose solution is similar to commonly consumed soda beverages.
- The glucose solution is well-defined and can be standardized on an international basis.
- Normal values already exist for both males and females which are corrected based on body weight. (See normal values listed at the end of this section).
- Average gastric half-emptying time clinical reasonable. (60-80 minutes)
- Since this is not a solid meal, there is no variation in gastric emptying due to variable amounts of chewing of the meal, between subjects or the same subject at different times.
- Labeling stability is not a issue because the 99mTc-SC particles are evenly distributed throughout the liquid solution.
- Solution distributes more evenly, making performance of geometric mean less critical for clinical work (but also still recommended for research studies)
- As yet, there are no definite comparisons to other gastric emptying meals with regard to pathologic conditions, although comparative research is currently underway.
- Since this is not a solid meal, it does not test the antrum's ability to grind food into small particles. The importance of this for routine gastric emptying pathologies has not been established.
Ideally, the exam should be performed in the morning after an
overnight fast [16]. If this is not possible, patients should
fast for at least 6-8 hours [16]. The patient may take
medications with some water on arising before coming for the
test [16]. Tobacco and alcohol should be withheld for at least
24 hours (smoking has been shown to slow gastric emptying) [12].
Other authors recommend patients refrain from smoking in the
morning of the test and throughout the time of imaging [16,17].
Mediactions such as prokinetic agents, antisecretory drugs,
gastric acid suppressors, and narcotics can all affect gastric
emptying [17]. Medications that can interfere with gastric
emptying should be discontinued for 3 days or 6-10 half-lives of
the drug [20]:
1- Narcotic/opiate analgesics can delay emptying and should be
discontinued 2 days before the test [demerol (merperidine),
codeine, morphine, oxycontin, percodan, percocet] [17]
2- Anticholinergics/amtispasmodics should usually be stopped 2
days prior to the test [Bentyl (dicyclomide), Donnatal, Levsin
(hyoscyamine), Robinul (glycopyrrolate)] [17].
3- Antidepressants; calcium channel blockers; gastric acid
suppressants; and aluminum containing antacids [12].
4- Prokinetic medications that enhance emptying such as
metoclopramide (Reglan), tegaserod (Zelnorm), erythromycin, and
domperidone (Motilium) should also be discontinued at least 2
days prior to the test [16,17].
6- Hyperglycemia can delay gastric emptying - diabetics should
have a fasting glucose level < 275 mg/dL [16,19]. At the time
the meal is ingested, diabetic patients should self-administer
their insulin at a dose that is generally half of what they
normally take [16].
Serotonin receptor (5-HT-3) antagonists such as ondansetron,
have little effect on GE and can be given for severe symptoms of
nausea and vomiting before performance of the exam [16].
The gold standard label has been considered to be radiolabelled chicken liver, due to its stability. The excellent stability occurs due to the intracellular location of the 99mTc-SC after phagocytosis by the macrophages. This technique is not practical in most locations because most nuclear medicine departments cannot keep live chickens.
Patients shouold fast for 4-6 hours prior to the exam [19. The
standard meal for gastric emptying should consist of 4 oz (120
gm) egg-white meal (Egg Beaters or equivalent) radiolabeled with
0.5-1 mCi of 99mTc-SC, two slices of toasted white
bread, 30 gm of strawberry jam, 120 mL water [16,19]. The total
energy of the meal is 255 kcal (72% carbohydrate, 24% protein,
2% fat, and 2% fiber) [17].
An allergy to egg is a contraindication to the meal [16]. A
sandwich is made of the egg meal and toast and should be
consumed in 10 minutes [16,17]. The patient should consume the
entire meal, but if this is not possible, at least 50% of each
component should be consumed [16]. If only a small portion of
the meal is ingested, the study cannot be considered diagnostic
as the results may overestimate the rate of gastric emptying
[17]. The 99mTc-SC egg label is relatively stable,
with about 80% still bound to the egg at 3 hours.
Another gastric emptying semi-solid meal is milk and cornflakes with sugar, but standardized emptying times would need to be established. Patients who cannot tolerate the egg-based solid meal may be tested with the nutritional supplement Ensure PLUS [20]. A rice-based solid meal substitute that is gluten free and vegan has documented normal values, but may not be widely available [20]. Radiolabeled pancakes have also used. Because emptying time can be affected by the content of the meal, the most important factor is to standardize the meal so that a reproducible response can be established [12].
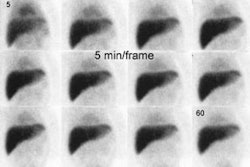
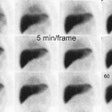
Previously, a continuous data acquisition was performed (64 x
64 matrix) with a frame rate of 30 to 60 seconds per image for a
duration of at least 90 minutes [12]. When using a single head
camera, images are acquired over the stomach in the LAO
position. After its ingestion, solid food remains for a short
period of time in the fundus, which distends to accommodate the
meal. The gastric antrum is more anterior than the gastric
fundus. As food moves from the fundus toward the antrum over
time, the measured gastric activity may actually appear to
increase as the bolus moves closer to the detector (less
attenuation). LAO imaging helps to reduce this geometric
problem. Alternatively, simultaneous anterior and posterior
images can be performed with the patient supine and a geometric
mean emptying determined [11].
The lag phase is more accurately measured using a geometric mean as the lag phase may appear longer on LAO imaging [13]. Data can also be recorded as static images every 10 or 15 minutes, although lag phase data cannot be recorded when using this method [12]. The normal time for the stomach to empty halfway is about 77 minutes +/- 32 minutes (mean +/- 2 SD) for a solid meal [10]. Values vary with gender, time of day, body position, and physical activity [10]. Other authors have reported that a percent gastric emptying of less than 34% at 90 minutes is abnormal [11]. The lag phase can also be recorded as the time to the point when duodenal activity is first identified [12]. Occasionally the stomach may appear to "refill" on time activity curves, and this is usually secondary to superimposition of activity within small bowel loops.
A simplified 4 hour protocol has been developed in which upright spot images (anterior and posterior simultaneous 1 minute acquisition) are acquired at 1, 2, and 4 hours and a decay corrected geometric mean determined ([anterior counts x posterior counts]1/2) [14,16,17,19]. This is presently the protocol recommended by the Society of Nuclear Medicine [16]. Between imaging, patients are permitted to sit in a designated waiting area and to walk to and from the imaging room [16]. Strenuous activity (climbing stairs, other physician visits, or other diagnostic tests) are to be discouraged [16]. Delayed gastric retention is defined as 90% retained at 1 hour, 60% at 2 hours, 30% at 3 hours, and 10% at 4 hours (normal percent gastric retention is 37-90% at one hour, 30-60% at 2 hours, and 0-10% at 4 hours) [14,16]. The additional imaging at 4 hours can identify patients with delayed emptying that may have had normal emptying times at 90 minutes [14,16]. Grading the severity of delayed gastric emptying has been suggested using 11-15% retained at 4 hours as mild delay, 16-35% as moderate delayed, and over 35% as severe retention [16]. Rapid gastric emptying is suggested when there is less than 30% retention at one hour [16].
Liquid water emptying exam
It has recently been suggested that combining a liquid water emptying study with a solid meal exam can identify additional patients with abnormal gastric emptying [15]. Gastroparesis can be detected following ingestion of clear liquid in up to 24-33% of patients with normal solid emptying exams [15,19]. Derangement in clear liquid emptying may indicate fundal dysfunction as the etiology for the patients gastroparesis [15].
For the exam, the patient drinks 300 mL of cool water with 0.2 mCi of Tc-SC or 0.1 In-111 DPTA (if the same day as a solid meal exam) in a semiupright (45 degrees) position [15]. Planar imaging is performed in the LAO projection using 1 minute per frame for 30 minutes [15]. Measurements of gastric emptying of liquids may not require geometric mean imaging [4]. This is thought to be due to a more even distribution of liquids throughout the stomach. Normal clear liquid emptying should be less than 19 minutes (mean +/- 2SDs) [15]. For a liquid only gastric emptying exam, a half-emptying time of more than 25 minutes is considered abnormal [21]. If the liquid emptying exam is to be performed concurrently with a solid emptying exam, the patient drinks 120 ml of water labeled with 0.1 mCi of In-111 DTPA with their egg meal [21]. For a combined liquid and solid meal exam, less than 39% emptying at one hour is considered abnormal [21].
A significant body of research has been done with gastric emptying of a glucose meal. The glucose meal has been shown to have high reproducibility in the same subject on repeat testing. The particular glucose meal used in our experiments is an 11% glucose solution, containing 50 grams of glucose in 450 mls of water. This has an osmolality of 662 mmol, a kcal content of 200 kcal and lemon-lime flavor similar to the 7-Up soda drink.
Advantages of the Glucose Meal
Disadvantages of the Glucose Meal
Normal Values for the Liquid Glucose Meal
The following normal values are for a 50 gram glucose meal (50 grams of glucose in 450 ml of water, as described above). Mixed group of49 subjects, 22 male and 27 female.
| Percent of glucose meal remaining in the stomach | ||
|---|---|---|
| Time (min) | % remaining | Std Dev |
| 0 | 100 | |
| 15 | 84.3 | 5.2 |
| 30 | 70.8 | 5.1 |
| 45 | 58.7 | 5.2 |
| 60 | 47.9 | 5.2 |
| 75 | 39.0 | 4.5 |
| 90 | 30.7 | 3.9 |
| 105 | 24.6 | 3.2 |
| 120 | 19.9 | 2.2 |
| T1/2 | 62.7 | 5.2 |
Gastric emptying exam findings:
Etiologies of delayed gastric emptying:
Diabetic neuropathy: Gastric motility is abnormal in approximately 25% of diabetic patients with autonomic neuropathy. Diabetic subjects with elevated blood glucose levels have been shown to empty more slowly than diabetic subjects with lower blood glucose levels [5]. There will be delayed gastric emptying with a prolonged lag phase [12].
Gastric outlet obstruction: Peptic ulcer, Neoplasm
Post surgical (Vagotomy): Of note, gastroplasty for the treatment of obesity does not appear to affect gastric emptying.
Scleroderma: Gastric involvement is seen in about 50% of patients with GI disease.
Anorexia nervosa: The delayed emptying is generally not as severe as that associated with diabetic gastroparesis.
Nausea: Nausea can markedly delay gastric emptying. This may be due to a reflex vagal reaction.
Billroth II: These patients typically demonstrate very early rapid emptying of the liquid phase of the meal, followed by little or no emptying of the solid component or the remaining liquid phase for a prolonged period. The rapid liquid emptying may give rise to symptoms of a dumping-type syndrome. Some patients demonstrate an early rapid emptying of both the solid and liquid components of the meal, followed by delayed emptying of both.
Drugs: Nicotine, Calcium Channel Blockers, Levodopa
Accelerated gastric emptying
S/P pyloroplasty: There is decreased resistance to emptying and large particles may pass directly into the small intestine. Vagotomy is often performed in conjunction with this procedure to decrease gastric motility.
Vagotomy: There may be decreased "receptive relaxation" of the fundus which permits food to more rapidly exit the stomach, despite the decreased motility.
Z-E syndrome
Drugs: Metoclopramide- stimulates gastric contractions and has a central antiemetic effect.
Hyperthyroidism
Malabsorption syndromes
Diabetic subjects without autonomic neuropathy: Several recent studies [6,7] have shown that at least some diabetic subjects (having either Type I or Type II diabetes) have rapid gastric emptying of calorie containing liquids and high carbohydrate solids such as pancakes [8].
REFERENCES:
(1) J Nucl Med 1995; Heyman S, et al. Radionuclide studies of the upper gastrointestinal tract in children with feeding disorders. 36(2):351-4
(2) Gastroenterology 1983; Brener et al. Regulation of the gastric emptying of glucose. 85: 76-82
(3) J Nucl Med 1991; Phillips et al. Linear gastric emptying of hyperosmolar glucose solutions. 32: 377-381
(4) Eur J Nucl Med 1995; Phillips WT, et al. Anterior, posterior, left anterior oblique, and geometric mean views in gastric emptying studies using a glucose solution. 22:154-157
(5) Diabetologia 1989; Horowitz et al. 32:151-159
(6) J Nucl Med 1992; Phillips WT, et al. Rapid gastric emptying of an oral glucose solution in type 2 diabetic patients. 33:1496-1500
(7) Gastroenterology; Frank et al. 109: 755-765
(8) Diabet Med. 1993; Schvarcz E, et al. Hypoglycaemia increases the gastric emptying rate in patients with type 1 diabetes mellitus. 10(7):660-3
(9) J Nucl Med 2000; Bestetti A, et al. 99mTc-sulfur colloid gastroesophageal scintigraphy with late lung imaging to evaluate patients with posterior laryngitis. 41: 1597-1602
(10) Radiographics 2002; Saremi F, et al. Pharmacologic interventions in nuclear radiology: indications, imaging protocols, and clinical results. 22: 477-490
(11) J Nucl Med 2004; Ziessman HA, et al. Standardization and quantification of radionuclide solid gastric emptying studies. 45: 760-764
(12) J Nucl Med 2004; Mariani G, et al. Radionuclide gastroesophageal motor studies. 45: 1004-1028
(13) J Nucl Med 1991; Maurer AH, et al. Comparison of left anterior oblique and geometric mean gastric emptying. 32: 2176-2180
(14) J Nucl Med 2007; Ziesman HA, et al. Experience with a simplified, standardized 4-hour gastric-emptying protocol. 48: 568-572
(15) J Nucl Med 2009; Ziessman HA, et al. The added diagnostic value of liquid gastric emptying compared with solid emptying alone. 50: 726-731
(16) Am J Gastroenterol 2008; Abell TL, et al. Consensus
recommendations for gastric emptying scintigraphy: a joint report
of the American neurogastroenterology and motility society and the
society of Nuclear Medicine. 103: 753-763
(17) J Nucl Med 2015; Maurer AH. Gastrointestinal motility, part
I: esophageal transit and gastric emptying. 56: 1229-1238
(18) J Nucl Med 2018; Orthey P, et al. Intragastric meal
distribution during gastric emptying scinitgraphy for assessment
of fundic accomodation: correlation with symptoms of
gastroparesis. 59: 691-697
(19) AJR 2018; Solnes LB, et al. Nuclear scintigraphy in
practice: gastrointestinal motility. 211: 260-266
(20) J Nucl Med 2020; Maurer AH, et al. Appropriate use criteria
for gastrointestinal transit scintigraphy. 61: 11N-17N
(21) J Nucl Med 2021; Zeissman HA, et al. Experience with
esophagogastrointestinal transit scintigraphy in the initial 229
patients: multiple regions of dysmotility are common. 62: 115-122