CNS Vascular Imaging:
Acute CNS Ischemia/Infarction:
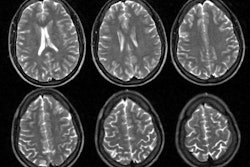
Tc99m-cerebral perfusion studies can be used to confirm the presence of cerebral infarction, monitor the effects of acute thrombolytic therapy, and to predict stroke outcome. A focal or regional area of hypo- or absent perfusion will be shown immediately after the event [12]. Cerebral SPECT is more sensitive than CT in the early (first 24 hours) detection of acute ischemia (sensitivity 88-95% vs. 20-63% for CT, MRI has a sensitivity of about 80% for the detection of acute infarction. The sensitivity of SPECT imaging is significantly reduced in the detection of lacunar infarctions). The defects noted on SPECT are also frequently larger than those noted on CT in about 50% of patients. This is because SPECT defects most likely represent a combination of a central zone of infarction which is surrounded by a penumbra zone of ischemia, but potentially viable tissue. Ipsilateral cortical diaschisis may also contribute to the size of the defect. On early images (obtained within 6 hours of the event), focal increased activity may be identified in up to 7% of patients within the infarcted cortex or ipsilateral basal ganglia on Tc-HMPAO imaging [15]. The focal area of hyperactivity is usually accompanied by a surrounding region of hypoactivity [15]. This finding may be related to spontaneous recanalization of occluded vessels [15].
During the subacute phase (between 1 to 5 and 14 days) the accuracy of SPECT decreases significantly and the size of the infarct may be grossly underestimated- particularly when using Tc-HMPAO as the imaging agent. This is mostly due to luxury perfusion which represents an uncoupling of flow and metabolism following an infarct possibly related to either local breakdown in the blood-brain barrier or hyperemia from local tissue acidosis. Luxury perfusion may mask the initial area of hypo- or absent perfusion with an area of apparently increased or normal tracer uptake, despite the absence of metabolism in the involved area [1]. Although hyperfixation can occur as early as 4 days after an acute infarction, it is more commonly observed 10 to 14 days afterward. Luxury perfusion is observed more frequently observed with Tc99m-HMPAO than with Tc-ECD or IMP. Even during the period of luxury perfusion, Tc-ECD images will demonstrate a persistent defect due to low retention of the tracer in the area of infarction as a result of altered esterase function in hypoxia (hypometabolism) which results in an inability to fix the agent intracellularly [10,11,13]. Tc-HMPAO fixation is not metabolically linked and therefore HMPAO imaging of subacute infarcts can demonstrate luxury perfusion [9]. By 30 days post event the hypoperfused area should easily be detected again [12].
Recent work has shown that local hyperperfusion occurs in some infarcts within 6 hours of the event. This finding may be related to early recanalization of the occluded artery with post-ischemic hyperemia (a result of vasodilatation in response to a decreased tissue pH). Unfortunately, early focal hyperemic lesions all progressed to areas of irreversible brain damage demonstrated by subsequent CT scans [2].
Diaschisis refers to a reduction in
neurometabolic activity
remote from the infarct site due to the disconnective effects of
the
infarct with loss of afferent signals to other regions of the
brain (in
other words, there
is loss of axons interconnecting the infarcted cortical regions
with
other brain
structures). Decreased cerebellar perfusion contralateral to the
cortical infarct (crossed
cerebellar diaschisis) is often noted during the acute and
subacute
phases of middle
cerebral artery territory strokes. Ipsilateral cortical diaschisis
can
also be seen and
can be difficult to distinguish from areas of actual infarction.
Areas
of decreased
activity secondary to diaschisis will demonstrate increased
activity
following the
administration of diamox due to the increased perfusion to these
areas
[3].
Differentiation of infarcted from neurometabolically inactive
tissue is
important as it
bears on stroke recovery prognosis and treatment protocols.
In patients with an acute stroke (under 6 hours since event), a perfusion defect less than 25% of the ipsilateral cortex has a good prognosis for recovery, and the risks of thrombolytic therapy in these patients may outweigh initiating no intervention. Defects over 75% of the ipsilateral cortex, however, are associated with significant morbidity, poor neurologic outcome, and mortality. Thrombolytic intervention in this setting may provide some degree of cerebral salvage [4]. Early imaging with defect quantification has also shown that areas which demonstrate a decrease of more than 40% in cerebral blood flow in comparison to the contralateral normal brain (Lesion/Contralateral side ratio below 0.6) are probably irreversibly damaged and these patients should not be candidates for reperfusion therapy. [2].
In patients with CNS arteriovenous malformations, an intracerebral "steal" phenomenon may produce relative ischemia in cerebral tissue adjacent to, or remote from the AVM. These areas can be detected by HMPAO imaging. The AVM itself typically appears as an area of reduced tracer activity.
Transient Ischemic Attacks:
Transient ischemic attacks occur in 10 to 20% of stroke patients. If no treatment is instituted following a TIA, about one third of these patients suffer a stroke within 5 years. The sensitivity of HMPAO imaging for identifying the area involved by the TIA declines with time, from about 60% in the initial 24 hours following the event, to less than 40% by 1 week [12].
Tc99m-HMPAO may help to identify a subset of patients with TIA's that require emergent intervention. Persistent focal defects on HMPAO examinations at 26 to 50 hours post event may be an indicator of impending complete infarction. In a limited number of patients, 75% of those who demonstrated such defects sustained an ipsilateral cerebral infarction in the following 3 to 7 days. Conversely, a normal study was not associated with impending infarction [4]
Acetazolamide (Diamox)?
challenge test is used to assess cerebral perfusion reserve.
The
exam may be useful in identifying CNS territory at risk in
patients
experiencing
TIA's and for patients being considered for carotid endarterectomy
or
ligation surgery (neurologic deficits occur in up to 5% of
patients
following carotid endarterectomy [18]). The exam may also be
useful as
a screening tool in asymptomatic patients.
Acetazolamide is a carbonic anhydrase inhibitor that causes an increase in cerebral CO2. This results in vasodilatation and increased flow in normal cerebral vessels. The increase in blood flow is dose dependent and ranges from an increase of 30-50% after I.V. administration of the agent [16]. In an area of reduced blood flow, where there has already been maximal vasodilatation (and thus loss of cerebrovascular reserve) there can be no augmentation of flow. Contraindications to the exam include cardiovascular instability, renal or hepatic insufficiency, and a history of allergy to sulfa drugs [16].
To perform the study, a baseline exam is compared to a SPECT
exam performed 25 minutes after the I.V. administration of 1 gm of
acetazolamide
given over 2 minutes (peak pharmacologic action is seen about
20-25
minutes after IV administration and the agent has a half-life of
about
90 minutes- blood flow returns to normal between
2-3 hours after injection) [12,16]. Areas of limited flow reserve
will
have decreased tracer
activity on the challenge exam compared to the baseline study [1].
A
decrease of 10 to 20%
in activity on the Diamox exam compared to baseline is considered
abnormal. The exam has
also been performed using both Tc99m-HMPAO and I-123 IMP which
permits
same day imaging
without moving the patient- thus the image slices from the 2
studies
should align
perfectly. Same day Tc99m-HMPAO protocols have also been
established
using a low dose (5
mCi) for the baseline exam, and 20 mCi for the challenge exam [3].
Diamox? is extremely
safe, the major side effect being a headache (the agent can induce
migraine headaches in
patients with a history of migraines), but cerebral steal is a
potential complication. However, other authors report other side
effects including metabolic acidosis, hypokalemia, numbness of the
extremities, tinnitus, GI disturbances, and Stevens-Johnson
syndrome
[18].
The challenge test is not without limitations. Up to 44% of patients with negative HMPAO challenge exams have been shown to have abnormal cerebral vascular flow reserve when evaluated by 15O-water PET [19]. Due to the nonlinear uptake properties of HMPAO and ECD, which underestimate perfusion in regions of increased flow, there can be limited contrast for flow differentiation. Additionally, acetazolamide has been shown to increase cerebral blood flow by only about 30% to 50% above baseline in normal older patients (range 5 to 70%). This is in contrast to coronary pharmacologic stress examinations in which coronary blood flow is increased by 300-400%. The limitations of a weak stress agent and a low contrast imaging agent help explain the low sensitivity for detecting significant (over 70%) stenoses. Generous cerebrovascular collaterals via the circle of Willis are another limitation of the exam [6].
Pre-operative evaluation for carotid endarterectomy:
Perioperative stroke can complcate carotid endarterectomy. Carotid shunting can be used to prevent stroke associated with hypoperfusion during carotid clamping [5]. However, shunting is not performed routinely as the procedure itself may be associated with complications such as clots within the shunt, embolization of air or atheromatous debris, and intimal injury.
Acetazolamide (ACZ) stress brain perfusion SPECT imaging may be useful in aiding in identification of patients that would most benefit from shunting [5]. Decreased perfusion following ACZ compared to a baseline exam reflects inadequate collateral blood flow and decreased vasodilatory capacity to maintain adequate cerebral blood circulation [5]. Patients with reduced cerebral vascular reserve (ie: decreased perfusion following ACZ) and significant contralateral ICA stenosis form a subgroup of patients most likely to require shunting during endarterectomy [5].
Hypoxic/Ischemic Encephalopathy of the Newborn:
Normally, in infants less than 3 months of age, thalamic perfusion is greater than cortical perfusion. As the child ages, there is orderly and progressive recruitment of telencephalic neocortical areas. In hypoxic encephalopathy, there is diffusely increased cortical activity within the first 24 hours after birth. By 7 days, this pattern will change and diffusely decreased cortical activity is then seen. Presently, thalamic hypodensities seen on CT scan are the best predictor of a poor outcome in these patients, however, for optimal sensitivity, CT scanning needs to be delayed until 72 hours after birth. Tc99m-HMPAO imaging may provide a more timely way to identify children in whom only minimal support is indicated due to their poor prognosis.
Brain Death:
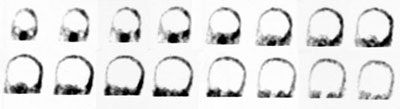
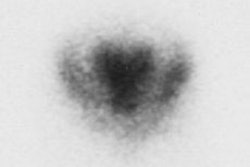
HMPAO is taken up by normal, viable brain tissue with no significant redistribution- this makes the agent particularly helpful in cases of suspected brain death. SPECT imaging in patients with brain death demonstrates no cerebral or cerebellar accumulation of the radiotracer. Additionally, as HMPAO is a perfusion tracer, it can be used to determine the viability of internal organs which may be used for transplants. [14]
|
Brain death: Coronal images from a Tc-HMPAO examination demonstrate no uptake of tracer by the brain parenchyma consistent with brain death. |
|
|
Cerebral Hemorrhage:
In patients with rupture cerebral aneurysms, there is a high morbidity and mortality in those patients that survive the initial insult secondary to vasospasm (with resultant ischemia) and recurrent bleeding. HMPAO imaging may aid in defining those patients with significant cortical vasospasm [7].
Preoperative Temporary Balloon Occlusion of the Internal Carotid Artery:
Patients with skull base or head and neck tumors may occasionally
require sacrifice of
an internal carotid artery if it is suspected
to be infiltrated by tumor. The complication rate from this
procedure
is relatively high
and preoperative identification of patients
at risk for cerebral infarction following the procedure is
essential.
Balloon
occlusion testing is performed to determine whether patients can
tolerate vessel
sacrifice. Unfortunately, studies have demonstrated that a
substantial
reduction in cerebral blood flow may occur during balloon
occlusion
without any significant neurologic
symptoms [17]. SPECT perfusion scintigraphy is more sensitive than
the
presence of neurologic symptoms in
identifying patients at risk for neurologic complications. In
fact,
neurologic symptoms
are present in only 11.5% to 28% of patients with severe perfusion
abnormalities [8].
Therefore, SPECT imaging can detect clinically silent decreased
cerebral
perfusion [17].
For the exam, a baseline study is performed and a second exam is then obtained during balloon occlusion. Occlusion is maintained for a 20-30 minute period. A cerebral perfusion tracer is injected 5 minutes prior to balloon deflation [12,17]. Tracer injection is performed sooner if patients begin to experience neurological symptoms. Focal or diffuse hypoperfusion, and the severity and magnitude of the perfusion defect are important parameters to consider in deciding whether the patient can tolerate carotid sacrifice surgery. Although no definitive quantitative criteria are available to define a perfusion abnormality, a difference in activity of 10% is generally accepted to be asymmetric [17].
An abnormal exam obtained during balloon occlusion is very useful in identifying patients who cannot tolerate vascular sacrifice (permitting a modification in their treatment plan). Unfortunately, a normal perfusion exam may not exclude the possibility of post-op neurologic complications and stroke rates up to 20% have been reported following a negative temporary balloon occlusion exam (probably the result of embolic or hemodynamic ischemic events) [8,17]. The poor predictive value of a negative exam may merely reflect the circumstances of the exam itself- which is usually performed under optimal conditions for only a short duration. The actual surgery is more complex and may result in hemodynamic alterations that can further compromise cerebral blood flow (or cerebral perfusion reserve) and result in neurologic complications. Overall, the predictive value of a negative SPECT exam for a satisfactory outcome after permanent carotid occlusion ranges from 80% to 100% [17].
The time of injection of the radiotracer may also theoretically
affect exam results.
Autoregulatory mechanisms within the brain
allow cerebral blood flow to return to baseline gradually over
several
minutes following
ICA occlusion. Tracer injections
performed within the first 2 minutes of the exam may more
accurately
reflect disturbances
in cerebral perfusion than injection
after 10 minutes of occlusion. An early injection, however, may
increase the number of
false positive exams [8].
REFERENCES:
(1) Radiol Clin North Am 1993; Van Heertum RL, et al. Spect brain
imaging in neurologic
disease. 31: 881-907
(2) J Nucl Med 1994; Shimosegawa E, et al. Cerebral infarction
within six hours of
onset: Prediction of completed infarction
with technetium-99m-HMPAO SPECT. 35: 1097-1103
(3) Nucl Med Ann 1994; Mountz J, et al. Brain Spect. 1-55
(4) Harvard Nucl Med Course, 94
(5) J Nucl Med 2001; Kim JS, et al. Acetazolamide stress brain-perfusion SPECT predicts the need for carotid shunting during carotid endarterectomy. 41: 1836-1841
(6) J Nucl Med 1994; Machac J, et al. Editorial: Cerebral versus
myocardial stress
perfusion imaging: Role of pharmacological
intervention in the diagnostic assessment of flow reserves.
35:
41-43
(7) Stroke 1990; 21: 252-59
(8) J Nucl Med 1996; Lorberboym M, et al. Brain perfusion imaging
during preoperative
temporary balloon occlusion of the
internal cartoid artery. 37: 415-420
(9) J Nucl Med 2001; Catafau AM. Brain SPECT in clinical practice. Part I: Perfusion. 42: 259-271
(10) J Nucl Med 1995; Moretti JL, et al. Cerebral perfusion imaging tracers for SPECT: which one to choose? 36: 359-363
(11) J Nucl Med 2001; Ogasawara K, et al. Dynamic and static 99mTc-ECD SPCT imaging of subacute cerebral infarction: Comparison with 133Xe SPECT. 42: 543-547
(12) J Nucl Med 2001; Camargo EE. Brain SPECT in Neurology and Psychiatry. 42: 611-623
(13) J Nucl Med 2001; Inoue Y, et al. Metabolism of 99mTc-ethylcysteinate dimer in infarcted brain tissue of rats. 42: 802-807
(14) Clin Nucl Med 1993; Wieler H, et al. Tc-99m HMPAO cerebral scintigraphy. A reliable, noninvasive method for determination of brain death. 18: 104-109
(15) J Nucl Med 2001; Sugawara Y, et al. Hyperactivity of 99mTc-HMPAO within 6 hours in patients with acute ischemic stroke. 42: 1297-1302
(16) Radiographics 2002; Saremi F, et al. Pharmacologic interventions in nuclear radiology: indications, imaging protocols, and clinical results. 22: 477-490
(17) J
Nucl Med 2002; Sugawara Y, et al.
Usefulness of brain SPECT to evaluate brain tolerance and
hemodynamic
changes during temporary balloon occlusion test and after a
permanent
carotid
occlusion. 43:1616-1623
(18)
J Nucl Med 2011; Sato Y, et al. Preoperative central
benzodiazepine
receptor binding potential and cerebral blood flow images on
SPECT
predict development of new cerebral ischemic events and cerebral
hyperperfusion after carotid endarterectomy. 52: 1400-1407
(19) J Nucl Med 2018; Acker G, et al. Brain
perfusion imaging under acetazolamide challenge for detection of
impaired cerebrovascular reserve capacity: positive findings
with 15O-water PET in patients with negative 99mTc-HMPAO
SPECT findings. 59: 294-298