Brain SPECT for the Detection of a Seizure focus:
Partial Complex Seizures/Temporal Lobe Epilepsy:
Epilepsy is one of the most prevalent neurological disorders-
affecting about 1% of the general population [10]. Seizures can be
classified as either partial (focal) or generalized [2]. Partial
seizures originate in a given area of the brain and can be divided
into simple (with no impairment of consciousness) and complex
(with impairment of consciousness) [10]. Both simple and complex
partial seizures may be preceded by sensations such as smells,
tingling, or buzzing [2].
About 10-20% of patients with partial complex seizures have
inadequate control on medical treatment [10]. Patients
unresponsive to anti-convulsant therapy may be surgical candidates
which can render the patient seizure free (surgical success rate
55-80% [19]) [2]. Scalp EEG often fails to accurately localize the
seizure focus and although depth EEG is much more accurate, it is
also extremely invasive and suffers from regional under sampling.
Most partial complex seizures originate in the temporal lobe. The
most common pathologic finding in these patients is mesial
temporal sclerosis which is thought to represent a gliotic scar.
Excision of this focus can lead to elimination of the seizures or
significantly improved pharmacologic control in 80% of patients.
Other etiologies include congenital or developmental malformation,
tumor, stroke, trauma, infection, vascular malformation,
meningoencephalocele, and phakomatosis [18].
Some authors suggest that CT and MRI have low sensitivity for
seizure foci detection, 17% and 34% respectively. However, other
authors indicate a very high sensitivity for the MR detection of
hippocampal (mesial temporal) sclerosis- with detection in up to
95% of cases [18]. Characteristics findings include T2
hyperintense gliosis in addition to atrophy and loss of normal
internal macrostructure of the involved hippocampus [18].
The role of brain SPECT is to localize the seizure focus [2].
Ictal Imaging:
Ictal imaging is the most sensitive exam for the identification
of epliptogenic seizure foci. The ictal exam requires that the
patient be placed in a special room with continuous video and EEG
monitoring. The patients medication is usually tapered off or
discontinued to increase the likelihood of a seizure episode [2].
The tracer to be used is placed by the patients bedside and is
already labeled. During the ictal phase of a complex partial
seizure, there is typically hyperperfusion of the mesial or
lateral aspects of the affected temporal lobe.
Tc99m-HMPAO of Tc-99m ECD injected during the ictal state or in
the immediate post-ictal period (within 30 to 60 seconds) will
show a focal area increased activity (hypermetabolic region) at
the seizure focus in 80 to 100% of patients. Some authors suggest
that late radiotracer injection (more than 45 seconds after the
seizure) are more likely to be associated with false or
non-localizing data [19]. Crossed cerebellar hyperperfusion can
also be identified in 75% of patients [10]. Ipsilateral or diffuse
cerebellar hyperperfusion may also be seen [11]. Ipsilateral basal
ganglia hyperperfusion is also common [11]. The injection should
be performed before the seizure becomes
generalized as identification of the seizure focus may not be
possible should this occur [10].
Interpretation of ictal exams requires a comparison to an
inter-ictal study [19]. Inter-ictal scans should be normalized and
subtracted from the ictal images to demonstrate areas of
hyperperfusion [19]. Ictal SPECT studies have reported
sensitivities between 81 to 93% [5,11] (sensitivity 89-97% for
temporal lobe epilepsy and 73-92% for neocortical epilepsy [13]).
The positive predictive value of SPECT imaging for localizing a
unilateral seizure focus can be as high as 97% (when the tracer
was injected immediately after the seizure) [9]. For localization
of seizure foci, ictal subtraction SPECT imaging has been shown to
be superior to interictal PET imaging (sensitivity 87% vs 56%)
[19].
Early post-ictal scans (injection within minutes [1 to 10 minutes] following seizure termination) can demonstrate mesial hyperperfusion and/or lateral temporal hypoperfusion on the side of the seizure focus. Such abnormalities are seen in about 70% of cases. The mesial hyperperfusion often has a crescent appearance and rarely persists for more than 6 to 8 minutes following the seizure, but can disappear as rapidly as 1 to 2 minutes. The associated lateral hypoperfusion often persists for longer periods of time (up to 20 minutes) and can be seen bilaterally, although it will be more pronounced on the side of the seizure focus.
Conventional image interpretation suffers from several limitations [11]. Side-by-side visual analysis is required to identify the seizure focus. The interpreting physician must visually account for slight differences in the patients head and image slice location when evaluating the exams [11]. Subtraction imaging of the ictal and inter-ictal exams can improve the sensitivity of SPECT imaging in localizing the seizure focus [11,12]. Generation of these subtraction images requires computer aided coregistration of the scans, quantitative normalization, and voxel-by-voxel subtraction [12]. Superimposition on MR images can also aid in improved spatial locatization [12].
Inter-ictal Imaging:
Following the seizure, there is relatively rapid progression
(generally within 20 minutes) to a hypoperfused state which
persists throughout the inter-ictal phase. Inter-ictal (seizure
free) SPECT studies have much lower sensitivity for the detection
of the seizure focus [72].
Continuous EEG monitoring should be performed for at least 2
hours before injection and for 15 minutes after injection to
exclude the possibility that a seizure has occurred [19].
Interictal FDG PET will demonstrate an area of diminished tracer activity at the seizure focus in up to 50% of patients. Inter-ictal PET FDG studies demonstrate a focal area of hypometabolism in 60 to 70% of patients with normal MRI's [1,2,3]. The area of hypometabolism is often much larger than the actual area of structural abnormality. It is difficult to assess the relationship of medical therapy to the finding of inter-ictal hypoperfusion. Anticonvulsants, especially phenytoin and phenobarbital, are known to reduce cerebral and cerebellar metabolism [4]. Up to 10% of patients may have a hypermetabolic focus identified on inter-ictal exams (which may appear hypometabolic on subsequent exams) [10]. Hypoperfusion of the ipsilateral thalamus can be seen in 26% of interictal exams [10].
Both HMPAO and ECD are equivalent for localization of a seizure focus [10,14]. The sensitivity for localization of the ictus site using inter-ictal SPECT scanning ranges from 40 to 66%. A meta-analysis of SPECT imaging for seizure focus localization revealed an inter-ictal sensitivity of 44%, a post-ictal sensitivity of 75% and an ictal sensitivity of 97% [14] (sensitivity of inter-ictal PET FDG imaging is about 79%). Incorrect localization is seen in 7.4% of interictal and 1.5% of postictal studies [14]. This may occur because rCBF in the seizure region is increased from its interictal value if the tracer is injected after subclinical seizure or during a time when there is an elevation in interictal spiking. The contralateral temporal lobe will then appear to have lower rCBF. Prognostically, patients with normal SPECT findings in the face of a localizing EEG are at a higher risk for a poor surgical outcome [4]. A combination of hypoperfusion in the interictal study followed by hyperperfusion in the same region on the ictal exam has absolute specificity [10]. Identification of perfusion asymmetry on interictal studies can be improved with the use of quantitative analysis [16].
|
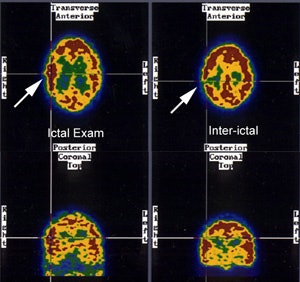
Seizure focus: Ictal SPECT perfusion exam demonstrates a hyperperfused (metabolic) area in the right temporo-parietal region which corresponds to a hyoperfused region on the inter-ictal exam. (Click image to enlarge) |
|
|
|
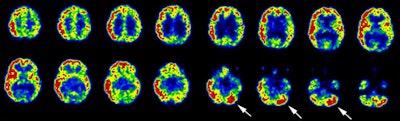
Crossed cerebellar hyperperfusion (diaschisis): The ictal-SPECT exam below demonstrated extensive hyperperfusion (metabolism) involving a large portion of the right cerebral hemisphere. Crossed cerebellar hyperperfusion (white arrows) was also demonstrated on this exam due to neuronal connections between the right brain and left cerebellum. |
|
|
The benzodiazepine (BN) receptor is a marker of the GABA-BN complex which is believed to be the main mediator of neuronal inhibition. GABA and BN receptor antagonists have been shown to induce seizures. C-11 flumazenil (C-11 FLU) is a benzodiazepine antagonist with high affinity for the central type benzodiazepine receptor that forms part of the GABA receptor. Both PET and SPECT studies have shown reduced BN receptor binding exclusively in the epileptic focus in patients with partial epilepsy, even when the cerebral perfusion exam is normal. [4] C-11 carfentanil studies of patients with partial complex seizures have demonstrated increased opiate receptor binding in the temporal lobe containing the epileptic focus (corresponding to the area of decreased glucose utilization identified on FDG imaging).
Frontal Lobe Epilepsy:
In the evaluation of frontal lobe epilepsy, SPECT images performed following the injection of Tc99m-HMPAO during the ictal period, demonstrated a hypermetabolic/hyperperfused seizure focus in 90% of cases. Hyperperfusion of the ipsilateral basal ganglia (73%) and contralateral cerebellum (64%) were also identified. Contralateral cerebellar hyperperfusion was primarily associated with seizure foci located in the motor cortex or frontopolar regions. Inter-ictal SPECT imaging detected a hypoperfused seizure focus in only 11% of patients and therefore, interictal scanning is probably only useful in serving as a baseline exam. [3]
Status Epilepticus:
Status epilepticus is a condition in which seizures occur either
continuously or so frequently that patients do not return to their
baseline state between seizures. Although EEG can be very useful
in the diagnosis, EEG abnormalities may be subtle or absent in
these patients. In the evaluation of partial status epilepticus,
ictal SPECT studies have demonstrated focal hyperperfusion in
areas concordant with that suggested by EEG. In patients without
status, no areas of hyperperfusion were detected. Status
epilepticus produces long term changes in regional cerebral blood
flow that are not evident following a single seizure. As a result
of this, persistent hyperperfusion may be observed for a prolonged
period of time (possible out to 6 days following the event). [7]
Epilepsy Syndromes:
Tuberous sclerosis: Epilepsy develops in 80-90% of TS patients
(cortical tubers are the epileptogenic focus/foci), in some as
early as infancy, and the epilepsy becomes intractable in 50-80%
of cases [17]. Typically, cortical tubers are seen as multifocal
areas of glucose hypometabolism on FDG imaging [17]. The tubers
are felt to be hypometabolic due to the decreased number of
neurons and simplified dendritic pattern within the tubers [17].
Imaging with 11C-AMT (11C-alphamethyl-L-tryptophan)
will show increased tracer concentration in epileptogenic tubers
during interictal imaging [17]. The increased tracer uptake
appears to be due to the activation of the kyurenine pathway,
leading to production of quinolinic acid, a preconvulsant, in the
tuber and adjacent dysplastic cortex [17].
REFERENCES:
(1) J Nucl Med 1993; Henry T, et al.
Clinical evaluation of interictal fluoring-18-fluorodeoxyglucose
PET in partial epilepsy. 34: 1892-1898
(2) J Nucl Med 1993; Newton M, et al. Ictal SPECT using technetium-99m-HMPAO: Methods for rapid preparation and optimal deployment of tracer during spontaneous seizures. 34: 666-670
(3) Neurology 1993; Harvey AS, et al. Frontal lobe epilepsy: clinical seizure characteristics and localization with ictal 99mTc-HMPAO SPECT. 43: 1966-80
(4) J Nucl Med 1994; Devous M. Editorial: The role of SPECT brain imaging in epilepsy. 35: 1094-96 (No abstract available)
(5) Radiol Clin North Am 1993; Van Heertum RL, et al. SPECT brain imaging in neurologic disease. 31: 881-907
(6) Nuclear Medicine Annual 1994; Mountz JM, et al. Brain SPECT: 1994 Update: 1-54 (p.35) (No abstract available)
(7) J Nucl Med 1994; Tatum WO, et al. Technetium-99m-HMPAO SPECT in partial status epilepticus. 35: 1087-1094
(8) J Nucl Med 2000; Lewis PJ, et al. Does performing image registration and subtraction in ictal brain SPECT help localize neocortical seizures? 41: 1619-1626
(9) J Nucl Med 2001; Catafau AM. Brain SPECT in clinical practice. Part I: Perfusion. 42: 259-271
(10) J Nucl Med 2001; Camargo EE. Brain SPECT in Neurology and Psychiatry. 42: 611-623
(11) J Nucl Med 2001; Shin WC, et al. Ictal hyperperfusion of the cerebellum and basal ganglia in temporal lobe epilepsy: SPECT subtraction with MRI coregistration. 42: 853-858
(12) J Nucl Med 2002; Boussion N, et al. Automated detection of local normalization areas for ictal-interictal subtraction brain SPECT. 43: 1419-1425
(13) J Nucl Med 2003; Sojkova J, et al. Does asymmetric basal ganglia or thalamic activation aid in seizure foci lateralization on ictal SPECT studies. 44: 1379-1386
(14) J Nucl Med 1998; Devous MD, et al. SPECT brain imaging in epilepsy: a meta-analysis. 39: 285-293
(15) J Nucl Med 2004; Oommen KJ, et al. The relative localizing value of interictal and immediate postictal SPECT in seizures of temporal lobe origin. 45: 2021-2025
(16) J Nucl Med 2005; Aubert-Broche B, et al. Evaluation of
methods to detect interhemispheric asymmetry on cerebral perfusion
SPECT: application to epilepsy. 46: 707-713
(17) J Nucl Med 2013; Kumar A, Chugani HT. The role of
radionuclide imaging in epilepsy; part 2: epilepsy syndromes. 54:
1924-1930
(18) AJR 2014; Friedman E. Epilepsy imaging in adults: getting it
right. 203: 1093-1103
(19) AJR 2021; Ponisio MR, et al. The role of SPECT and PET in
epilepsy. 216: 759-768