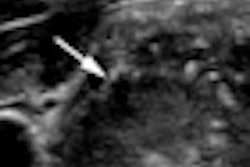
ATLANTIC CITY, NJ - The lack of approval of radiology ultrasound contrast in the U.S. has long stuck in the craw of the technology's advocates. But clinical trials are now moving forward with hopes of rectifying the situation, according to a presentation at the annual Leading Edge in Diagnostic Ultrasound conference.
Ultrasound contrast agents are commonly used in Europe for both radiology and cardiology applications. For example, one of the more popular agents, SonoVue from Bracco of Milan, Italy, began became commercially available in Europe in 2001, and as of last year, the company had shipped more than 1 million vials.
Ultrasound contrast has navigated a trickier road in the U.S., however, where the technology has only been available for cardiology use. Ultrasound contrast also suffered a setback in 2007, when safety concerns prompted the U.S. Food and Drug Administration (FDA) to slap a black box warning on the two agents on the U.S. market for cardiology applications: Definity from Lantheus Medical Imaging of North Billerica, MA, and Optison from GE Healthcare of Chalfont St. Giles, U.K.
Subsequent clinical studies have largely laid to rest these concerns, demonstrating that patients who receive ultrasound contrast for echocardiography applications do not have increased risk of death or myocardial infarction during short-term or long-term follow-up. But the damage was done, and several contrast developers postponed their plans to bring new agents to market for either radiology or cardiology applications.
Tide begins to turn
But the tide appears to be turning. The FDA relaxed its black box warning in 2008, and vendors are now working with clinicians to develop the clinical trials required to bring new products to market.
In the case of SonoVue, patient recruitment is now under way for two phase III clinical trials to investigate the utility of the agent for focal liver lesion characterization, and other companies have also expressed interest in pursuing clinical trials for radiology applications in the U.S., according to Dr. Barry Goldberg, a professor of radiology at Thomas Jefferson University Hospital in Philadelphia and a clinical investigator in one of the trials.
"My prediction is that the routine use of [ultrasound contrast agents] will improve patient care through more accurate and cost-effective diagnoses of a variety of abnormalities," Goldberg said.
A multicenter study has been initiated at sites in the U.S., Canada, and China in support of a regulatory submission to the FDA. The study involves the initial enrollment of up to 10 subjects at each site to serve as training cases for the characterization of focal liver lesions (FLL). Data from these cases will be included only in the safety analysis, Goldberg said.
Ultimately, 156 subjects will be enrolled to obtain 147 evaluable subjects, of whom more than half are anticipated to have a malignant lesion. The two multicenter trials (called BR1-128 and BR1-130) feature competitive enrollment and a minimum of 10 and a maximum of 30 subjects per site. The first patient was enrolled in September 2009, with enrollment expected to be completed in September 2010.
The primary objective is to demonstrate that the sensitivity and specificity of SonoVue-enhanced ultrasound is superior to that of unenhanced ultrasound for characterizing benign versus malignant focal liver lesions using a final diagnosis based on histology or combined imaging (contrast-enhanced CT and/or contrast-enhanced MRI) and clinical data as the truth standard, Goldberg said.
As secondary objectives, the investigators are also seeking to:
- Evaluate the accuracy and other performance parameters (such as positive and negative predictive value) of SonoVue-enhanced ultrasound for characterizing benign versus malignant FLL compared to unenhanced ultrasound
- Evaluate the ability of SonoVue-enhanced ultrasound to obtain a specific diagnosis of FLL compared to unenhanced ultrasound
- Evaluate the inter-reader agreement in the assessment of unenhanced and SonoVue-enhanced ultrasound images
- Provide evidence of safety and tolerability of intravenous administration of SonoVue in subjects with focal liver disease
Both trials are also the first to adhere to all of the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Study Group guidelines for the use of contrast agents in ultrasound, the American Association for the Study of Liver Diseases (AASLD) practice guidelines for managing hepatocellular carcinoma, and the American Institute of Ultrasound in Medicine (AIUM) 2007 recommendations for contrast-enhanced liver ultrasound imaging clinical trials, Goldberg said.
Sixteen sites will participate in the BR1-128 trial, including 14 in the U.S. and two in China. Nine are actively enrolling patients at this point, Goldberg said. Twelve sites in North America have indicated they will participate in BR1-130, with three more planned in China.
Trial protocol
In the trials, SonoVue will be administered intravenously into an upper extremity vein as a 1.4-mL bolus injection with a 20-gauge catheter. Immediately following injection, a 5-mL to 10-mL saline flush will be administered.
A maximum of two injections are allowed under the trials' clinical protocol, Goldberg said. The first injection will be used to assess the dynamic enhancement profile of the contrast agent within the target lesion and to the surrounding parenchyma for characterization. A second 2.4-mL dose may be injected in cases of technical failure of the first bolus or for characterizing a second liver lesion.
An interval of 30 minutes must follow the first SonoVue administration before the second administration, he said. Both unenhanced ultrasound and SonoVue-enhanced ultrasound will be performed in the same session, using commercially available ultrasound systems by qualified personnel under physician supervision.
Diagnosis of the target lesion must be confirmed by biopsy/surgery of the lesion performed from 24 hours to 30 days after SonoVue administration; if biopsy/surgery is not planned, then both a contrast-enhanced CT and contrast-enhanced MRI must be performed from 24 hours to 30 days after SonoVue administration (unless already performed from 30 days to 48 hours prior to SonoVue administration), according to Goldberg.
"Any target lesion 1 cm or smaller must have biopsy or surgery performed as the truth standard," he said.
Subjects who do not have the protocol-required truth standard must be followed up for at least six months to document the progression or nonprogression of disease as determined by CT or MRI.
Focal liver lesions will be characterized using the European guidelines for contrast-enhanced ultrasound of the liver.
By Erik L. Ridley
AuntMinnie.com staff writer
May 13, 2010
Related Reading
New CEUS technique images angiogenesis, March 1, 2010
Experience counts when reading contrast ultrasound liver images, January 19, 2010
Interest grows in noncardiac ultrasound contrast applications, May 21, 2009
CEUS of focal liver lesions in the noncirrhotic liver, May 19, 2009
Bracco revisits plans for U.S. launch of SonoVue, March 8, 2009
Copyright © 2010 AuntMinnie.com