Surgery can sometimes help patients with their Parkinson's disease symptoms when medications are ineffective. But could transcranial MR-guided focused ultrasound (MRgFUS) offer a better and more noninvasive treatment alternative? Researchers at the University of Virginia are taking the first step toward answering that question.
The institution recently launched a phase I clinical trial to evaluate the safety and effectiveness of MRgFUS in reducing tremor related to Parkinson's disease. The first two patients in the trial have already been treated.
"This is a pilot study, so our primary efficacy measure is safety and adverse event recording, but we will also collect efficacy data that has to do with tremor and Parkinson's symptoms and quality-of-life measures," said principal investigator Dr. Jeffrey Elias, an associate professor of neurological surgery and neurology in the university's department of neurosurgery.
He spoke during a recent press conference sponsored by the Focused Ultrasound Foundation, which is contributing funds for the trial.
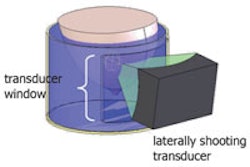
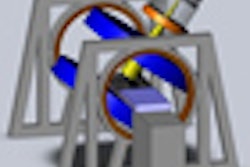
Deep brain stimulation, which involves drilling holes in the skull and placing a pacemaker system in the brain, is the current frontline surgical option in Parkinson's patients who are not responding to medication. MRgFUS, however, can noninvasively target a small area deep in the brain using MR-guided focused sound waves, according to the Focused Ultrasound Foundation.
No scalp incisions, electrodes, or general anesthesia are required, and patients can remain awake and communicative during the procedure, the foundation said. The organization believes the technology could be a significant improvement over the current standard of care.
The Parkinson's study follows a recently completed pilot clinical trial that was also performed by Elias at the University of Virginia. In that study, Elias and colleagues investigated the use of the ExAblate (InSightec) focused-ultrasound system in treating essential tremor. Tremor improvement was found in all 15 patients, and the researchers now plan to proceed to a large, multicenter, international trial.
Tremor-dominant Parkinson's
Based on the success of the essential tremor trial, the Virginia team has now turned its attention to Parkinson's disease, launching a prospective, randomized, double-blinded feasibility study focusing on patients with a tremor-dominant subtype of Parkinson's disease.
"This clinical trial is focused on treating tremor as the primary symptom," Elias said.
Parkinson's disease has several different subtypes. While approximately 75% to 100% of Parkinson's disease patients will have tremor at some stage, around 8% will have a pure tremor-dominant subtype of the disease. Tremor-dominant Parkinson's disease may present at a younger age and progress more slowly, according to Ellis.
To be eligible for inclusion in the study, patients must be older than 30 years and diagnosed with idiopathic Parkinson's disease with a tremor, with a postural instability and gait disorder (PIGD) score of at least 1.5. The tremor has to be severe, disabling, and refractory to the usual medications used with Parkinson's disease.
Patients with cognitive impairment (Montreal cognitive assessment score of 2 or less), Beck depression inventory score greater than 14, or other Parkinsonian symptoms are excluded from the trial.
Thirty patients are expected to be enrolled at the single-site study; 10 of the 30 will serve as the control group and receive a sham procedure without power from the transducers. Those in the control group will be able to cross over to the treatment arm of the study after three months.
Thorough cognitive assessments will be performed before and after the procedure to ensure that the treatment is safe, Elias said. Clinical and MRI measures will be tracked at one, three, six, 12, and approximately 24 months.
The Parkinson's trial is being sponsored a public-private partnership that includes the Focused Ultrasound Foundation, the Heller Foundation, the Commonwealth of Virginia, InSightec, and other donors.