Monitoring and understanding a patient's response to therapy plays a central role in optimizing cancer treatment. Advanced imaging technologies could enable monitoring of tumor response at an earlier stage in the treatment course, providing a means for personalized therapy adaptation.
At the American Association of Physicists in Medicine (AAPM) annual meeting in Charlotte, NC, "Imaging for Therapy Assessment" was the theme of the John S. Laughlin Science Council Research Symposium. Each year, this symposium addresses a chosen topic of particular relevance in medical physics research, this time highlighting the need to develop sensitive and specific quantitative imaging techniques as surrogate biomarkers for use in clinical trials and routine clinical practice.
Responder or not?
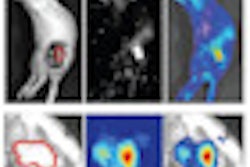
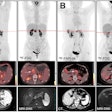
The session's first speaker was Hao Zhang, PhD, from the University of Maryland School of Medicine, who examined the application of FDG-PET for modeling the response of esophageal cancer patients to chemoradiotherapy.
The current standard of care for esophageal cancer is chemoradiotherapy followed by surgery. But, as Zhang explained, chemoradiotherapy responders have a good prognosis and may not require surgery, which increases mortality and morbidity rates. For nonresponders, on the other hand, surgery improves survival; thus it's vital to be able to distinguish between the two.
"Adding surgery after chemoradiotherapy is still a controversial topic," he said. "The purpose of our research was to develop tumor response models for locally advanced esophageal cancer patients, using extracted quantitative spatial-temporal FDG-PET features."
Zhang and colleagues examined data from 20 patients who underwent PET/CT prior to starting chemoradiotherapy and four to six weeks after its completion. All patients subsequently underwent surgery, providing access to histology results.
The team examined a range of potential image features for predicting tumor response, including conventional PET/CT response measures (such as SUVmax, SUVpeak, and tumor diameter); clinical and demographic parameters (such as tumor stage and histology, patient age, and sex); and spatial-temporal PET features, which characterize FDG uptake distribution, texture, geometric tumor properties, and temporal changes. Postchemoradiotherapy texture features, for example, revealed that responders exhibited homogenous FDG uptake, while nonresponders showed more heterogeneous uptake.
The next step was to identify relationships between the extracted spatial-temporal features and pathological response to determine an optimal subset of features. The researchers used logistic regression and support vector machine (SVM) models for prediction, with the optimal feature subset as input. They saw that the best tumor response prediction was obtained using SVM with 24 selected PET features: 10 intensity, 12 texture, and two geometric features.
This prediction model provided 100% sensitivity, with all chemoradiotherapy responders identified, and 90% specificity. This represents a significant improvement over conventional PET/CT response measures, which offered 57% specificity and 85% sensitivity (using SVM). "The ability to precisely predict pathological response to chemoradiotherapy will give physicians the confidence to make informed decisions on deferring or accelerating a patient's surgery," Zhang concluded.
Sensitive substructures
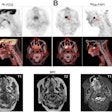
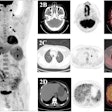
Next up, Jinsoo Uh, PhD, from St. Jude Children's Research Hospital, told the AAPM delegates about the use of MR diffusion tensor imaging (DTI) to assess brainstem injury in pediatric medulloblastoma patients receiving radiotherapy.
"The brainstem is the critical dose-limiting organ during treatment of childhood brain tumors, but not much attention has been paid to the effects of radiation on the brainstem," Uh said."It has been considered as a single organ, but different substructures may have different sensitivities."
Uh and colleagues examined 49 medulloblastoma patients (median age, 9 years), acquiring DTI data at baseline, completion of radiotherapy (approximately three months), and every six months thereafter. Patients underwent surgical resection, fractionated craniospinal irradiation, and chemotherapy. The researchers also examined 52 healthy volunteers to distinguish normal age-related changes.
The team then examined temporal changes in DTI-derived parameters such as fractional anisotropy, which reflects radiation-induced white-matter injury, and axial and radial diffusivity. Results were compared across brainstem substructures, including motor (corticospinal) tracts and sensory (medial lemiscus) tracts, and transverse pontine fibers (which are involved in coordination).
Temporal changes in DTI parameters were not always consistent between the different brainstem fiber tracts. For a demonstrated patient, fractional anisotropy in transverse pontine fibers decreased at 17 and 44 months after radiotherapy, while fractional anisotropy in the motor and sensory tracts was slightly decreased at 17 months but recovered to baseline at 44 months.
This decline in fractional anisotropy was often accompanied by decreased axial diffusivity and/or increased radial diffusivity, which are thought to indicate axonal degeneration and demyelation, respectively. "The transverse pontine fibers showed more significant changes, reflecting white-matter injury, than the corticospinal tract, medial lemiscus, and middle cerebellar peduncle," Uh explained.
He pointed out that this regional variation in temporal DTI changes was not due to different dose distributions, as the substructures received similar mean doses. The results therefore imply the existence of regional sensitivity to radiation, supporting the need for tract-based assessment in radiation treatment planning to minimize brainstem toxicity. The researchers now plan to correlate their results with neurological exams and to study patients with severe neurotoxicity.
Breast image registration
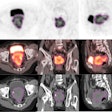
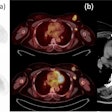
Roxana Vlad, PhD, from Princess Margaret Hospital, described the development of a procedure for registering breast whole-mount histology slices with multimodality images. The underlying motivation for this work was to develop imaging methods for early response assessment of invasive ductal carcinoma treated with neoadjuvant chemotherapy, using quantitative ultrasound, which can characterize cell death following therapy.
"Our hypothesis is that quantitative ultrasound based on the analysis of frequency-dependent information can distinguish responders from nonresponders at an earlier time point during treatment, enabling a personalized cancer-care approach," Vlad explained, presenting ultrasound data that clearly revealed significant differences in responders versus nonresponders.
To validate their approach, Vlad and coworkers needed to register the ultrasound images with dynamic contrast-enhanced MRI (DCE-MRI) (to identify residual tumor) and histology (as the ground truth representation). Thus they had to address the considerable deformations between DCE-MRI (prone), ultrasound (supine and under probe pressure), and histopathology (sliced, processed, reconstructed in a 3D volume).
The researchers recorded prone DCE-MRI and supine ultrasound images (using a freehand 2D probe with a tracking tool) at the start of chemotherapy and prior to breast resection. They also obtained ex vivo MRI scans of the breast before histological processing.
All image datasets were rigidly registered, the breast and tumor were contoured on each dataset, and the contours were converted to a volumetric mesh. Deformations between the imaging modalities were estimated using the ex vivo MRI scans, and then applying a biomechanical-based model deformation algorithm to calculate the in vivo to ex vivo deformation map.
Vlad presented an example in which the registration discrepancy between ultrasound and DCE-MRI images (at the center-of-mass of a necrotic structure) was reduced from 15 mm using rigid registration to 8 mm when applying the deformation map.
"We have developed a framework for predicting realistic breast deformation from MR prone to whole-mount histopathology and MR prone to freehand ultrasound," Vlad concluded. "Overall the registration differences reduced by 50%."
Hot topic
The abovementioned studies are just a selection from a total of nine presentations at the John S. Laughlin Science Council Research Symposium. Other speakers included Peter Scully, from the University of Wisconsin, who used fluoro-L-thymidine (FLT) PET to assess treatment response in patients with multiple tumors. Scully observed substantial intrapatient heterogeneity of tumor response. Intriguingly, response did not correlate with any size, SUV-based, or nonuniformity pretreatment metrics.
Quinn Matthews, PhD, from the BC Cancer Agency in British Columbia, discussed single-cell Raman spectroscopy for monitoring radiation response in human tumor cell lines. He found that radioresistant cells exhibited a strong Raman signature as early as two days after the start of treatment, which indicated the presence of radiation-induced glycogen. Radiosensitive cells exhibited a weak signature, with no contributions from glycogen, implying that Raman spectroscopy of radiation-induced glycogen synthesis could provide an early biomarker for radiation-resistant cancer.
When the theme of therapy assessment was first introduced to the AAPM annual meeting, it garnered little attention. Interest in this area has, however, grown at speed. And as this year's conference indicated, monitoring of therapeutic response and the associated imaging developments required to enable this look set to play a significant role in future attempts to optimize clinical outcomes.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.