The first talk saw David Kirsch from Duke University Medical Center (Durham, NC) describe how molecular imaging probes can be used to detect microscopic residual cancer during surgery. In particular, Kirsch discussed soft-tissue sarcomas, which are currently treated using radiotherapy and surgery. He noted that the use of surgery alone results in 68% local control, while adding radiotherapy improves control to 89%.
While these figures advocate the use of radiotherapy, they also imply that it's only really needed in a subset of patients. The ability to identify patients with residual disease after resection -- via optical imaging, for example -- could enable personalized sarcoma treatment: intensifying radiotherapy for those with residual cancer and avoiding it for those without.
Various research groups are developing optical imaging probes to do just that, says Kirsch. Many of these probes are based around near-infrared (NIR) fluorophores that are tailored to enter tumor cells and then fluoresce. Such probes can be used both to guide surgical resection and detect residual tumor cells.
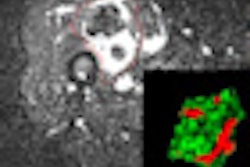
Kirsch described the use of an NIR probe called ProSense-680 in a mouse model of soft-tissue sarcoma. Mice were injected with the probe and surgery was performed 24 hours later, under the guidance of optical imaging. Initial experiments revealed that the sarcoma could be visualized and removed, and that the tumor bed could then be reimaged to examine for residual cancer.
Over the past couple of years, the Duke group has been working to increase the sensitivity of its optical imaging system, with the ultimate goal of mapping individual cancer cells to two to four pixels on a CCD detector. Kirsch notes the team has now achieved detection of single cancer cells. The team is currently undertaking a study to determine the relationship between the presence of fluorescence (indicating residual tumor) after surgery and ultimate clinical outcome.
"To date, all mice with residual fluorescence have developed local failure, and the majority of mice with negative residual fluorescence remained tumor free," he explained. The few recurrences in nonfluorescing regions were attributed to either threshold intensity issues, which the researchers are addressing via software optimization, or possibly to tumor heterogeneity.
Kirsch predicts that the first application of this technique in patients will be to identify those with residual tumor and subsequently intensify their radiotherapy. "One day in the future, if sensitivity and specificity are reliably high, we may be able to allocate patients to surgery alone based on this setting," he concluded.
Oxygen tracking
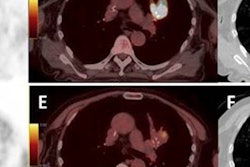
Imaging can also play an important role in tumor staging, using modalities such as PET or MRI to account for tumor biology. "If we try to use biological staging as a complement to TNM staging, we present ourselves with the opportunity for personalized treatment," David Brizel, from Duke University Medical Center, told ASTRO delegates.
Brizel proposed that hypoxia is "perhaps truly the prototype" for tumor staging, noting that for head and neck cancers at least, the survival probability is increased for patients with well-oxygenated tumors, regardless of their TNM stage. Various techniques are used to gauge hypoxia, including PET and functional MRI. Brizel pointed out that virtually all hospitals already have an MRI scanner, and many also have PET/CT available.
Brizel also told delegates how the ability to repeat noninvasive measurements such as MRI at several time points throughout therapy enables assessment of treatment response. Diffusion-weighted (DW) MRI, for example, which tracks the motion of water molecules through the tumor, can be used to follow changes in tumor density during treatment.
He described a study that showed how a change in absolute diffusion coefficient between pre- and midtreatment scans was associated with favorable outcome in the treatment of head and neck cancer. "Much work needs to be done on DW-MRI but preliminary data are encouraging," he said.
Another promising option is dynamic contrast-enhanced (DCE) MRI, which uses a parameter called Ktrans (related to the transfer of contrast between blood plasma and tumor) to determine vascular permeability. A study recording DCE-MRI at various time points before and during chemoradiation for head-and-neck cancer revealed that Ktrans decreased in patients who responded to treatment and increased in those who failed.
The Duke team is now working on a separate functional imaging trial using a combination of DCE-MRI, DW-MRI, and FDG-PET scanning during chemoradiation of head and neck cancers. The aim is to verify that the changes seen are induced by the treatment and not just due to tumor instability.
The final symposium speaker, Quynh-Thu Le from Stanford School of Medicine (Stanford, CA), took a closer look at the potential of PET imaging for radiotherapy planning and hypoxia imaging. She presented studies showing that PET tumor volumes are closer to surgical specimens than CT/MRI volumes. She also described a Stanford study showing that post-treatment FDG-PET is valuable as a prognostic factor, particularly in high-risk patients.
Le also presented data from hypoxia tracers, such as F-18 fluoromisonidazole (FMISO), that exhibit direct correlation between tumor oxygen and tracer uptake. She cautioned, however, that tumor hypoxia fluctuates both temporally and spatially in solid tumors. She described a study in which F-18 FMISO scans were performed three days apart with no intervention. Out of 13 patients, only half had correlation between the two scans.
"The concept of hypoxia imaging at a single time point to guide IMRT dose escalation may not be feasible," she concluded. "A novel hypoxia imaging approach is needed if dose escalation is contemplated."
By Tami Freeman
Medicalphysicsweb editor
November 13, 2009
Related Reading
Radiosensitive patients identified by genetic traits, September 25, 2009
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community Web site covering fundamental research and emerging technologies in medical imaging and radiation therapy.