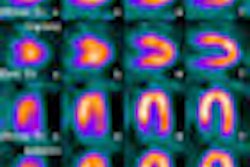
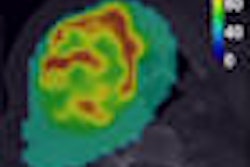
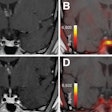
GE Healthcare of Chalfont St. Giles, U.K., has received clearance from the U.S. Food and Drug Administration (FDA) to market its AdreView (iobenguane I-123 injection) molecular imaging agent for the detection of rare neuroendocrine tumors in children and adults.
The FDA's ruling comes after the agency designated AdreView for priority review in June.
AdreView is designed to aid in the diagnostic assessment of pediatric cancer patients with neuroblastoma, the most common extracranial solid tumor in young children up to five years of age. It's also indicated for pheochromocytoma, a rare tumor that typically affects adults.
Both tumors usually arise from tissues of the sympathetic nervous system, most commonly in the adrenal glands. The tumors can be difficult to detect at an early stage because symptoms may be nonspecific when the tumors are small.
AdreView images reflect the functional behavior of tumor cells, thus allowing clearer characterization of small tumors in comparison to similar-appearing, but nonmalignant, tissues. AdreView's information is intended to complement anatomic imaging procedures, such as CT, MRI, and SPECT.
GE noted that AdreView also will permit imaging of these tumors with a lower radiation dose than other agents that have been available for the same purpose.
The company plans to begin shipping AdreView to hospitals and imaging centers in the U.S. in the coming weeks.
Related Reading
GE upgrades PACS-IW software, September 17, 2008
GE inks 7-telsa MRI deal with Varian, September 15, 2008
GE shows new Centricity product, September 11, 2008
GE debuts new ultrasound scanner, September 5, 2008
GE debuts ob/gyn US upgrades, August 27, 2008
Copyright © 2008 AuntMinnie.com