Two new studies evaluated three different MR contrast agents for brain and spinal imaging. The lead author for both papers was Dr. Kenneth Maravilla from the University of Washington in Seattle.
In the first study, Maravilla and a multi-institutional group compared two gadolinium-based contrast agents for the enhancement of central nervous system (CNS) lesions. In the second, Maravilla and his Seattle-based colleagues tested a half-life gadolinium-based contrast agent in functional MRI (fMRI).
MultiHance versus Magnevist
Higher doses of gadolinium-based contrast agents result in a significant improvement in contrast enhancement, but they also mean higher costs and greater patient exposure, thereby limiting the use of gadolinium-enhanced MR in the CNS, the authors of the first study explained. An alternative means to improve contrast is desirable, they stated.
To that end, the group compared a 0.1 mmol/kg dose of gadobenate dimeglumine (MultiHance, Bracco Diagnostics, Princeton, NJ) and 0.1 mmol/kg dose of gadopentetate dimeglumine (Magnevist, Berlex Imaging, Wayne, NJ) for MRI of CNS lesions.
This double-blinded, randomized, intraindividual crossover design study was sponsored by Bracco Diagnostics. Maravilla, and co-author Dr. Val Runge from Scott and White Memorial Hospital in Temple, TX, have consulted for Bracco. Co-author Dr. Leo Wolansky from University Hospital in Newark, NJ, received support from Berlex.
For this prospective research, 157 patients with suspected or known lesions of the brain or spine were enrolled in 11 participating centers during a one-year period. They were randomized to group A, who were first imaged with gadobenate and then again with gadopentetate. The order of contrast administration was reversed in group B.
All patients underwent MRI on a 1.5-tesla system with a standard protocol that included T1- and T2-weighted spin-echo (SE) acquisitions before contrast injection. T1-weighted SE imaging was done after contrast administration. Three independent neuroradiologists, who were not affiliated with the study centers, evaluated all the images, which were obtained in global-matched-pairs fashion.
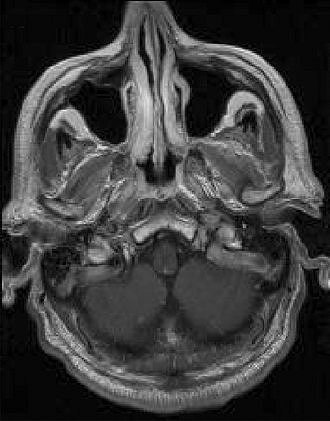 |
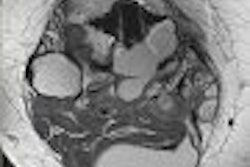
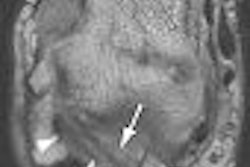
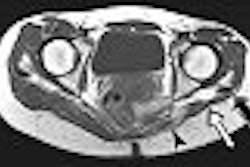
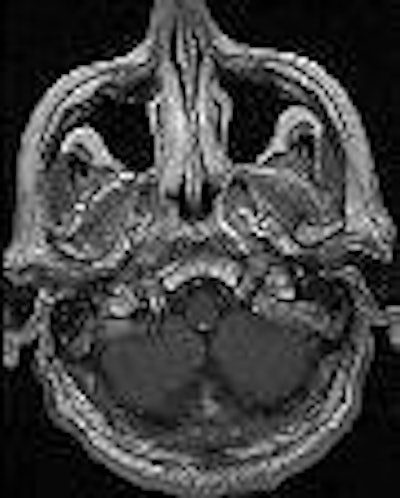
| Above, transverse T1-weighted SE MR image (590/12) in 61-year-old man with metastatic lung cancer after administration of 0.1 mmol/kg of gadopentetate reveals multiple enhancing lesions in right cerebellum and posterior medulla. Below, image acquired with identical parameters as previous image after administration of 0.1 mmol/kg of gadobenate reveals improved contrast enhancement of lesions seen with gadopentetate and unequivocal detection of two additional metastatic lesions (arrows) in right cerebellar hemisphere. |
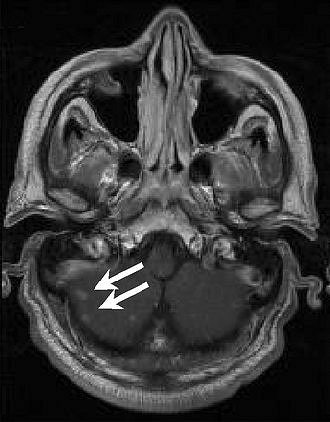 |
| Figure 2ab. Maravilla KR, Maldjian JA, Schmalfuss IM, et al, "Contrast Enhancement of Central Nervous System Lesions: Multicenter Intraindividual Crossover Comparative Study of Two MR Contrast Agents." Radiology 2006; 240: 389-400. |
The results were based on the exams of 151 patients. First, there was no significant difference in the distribution of CNS lesions between groups A and B. The readers expressed an "overwhelming" preference for gadobenate for lesion border definition (62.9% agreement), degree of contrast enhancement (62.9%), definition of extent of disease (72.8%), and visualization of internal morphologic features (60.3%).
Gadobenate was also preferred for gliomas, metastases, and meningiomas. Based on the quantitative evaluation, the lesion-to-brain or lesion-to-spine contrast-to-noise ratio was better with gadobenate. There was no difference between the two agents with regard to adverse events.
The global diagnostic preference for all the readers was 60.3%. "The greater diagnostic preference for one agent rather than the other was caused by the combination of superior contrast enhancement and better delineation of lesions and/or normal brain or of internal structures of the lesion," the authors wrote (Radiology, August 2006, Vol. 240:2, pp. 389-400).
Overall, there was an approximately 30% increase in quantitative lesion enhancement for gadobenate. The group attributed the preferential performance of gadobenate to its greater T1 relaxivity in the blood.
Commenting on the study, a representative from Berlex agreed with Maravilla's group that a limitation of the study was that it did not evaluate the potential effect of gadobenate on patient treatment and outcome.
Further investigations must determine optimal sequence parameters, as well as ways to fine-tune gadobenate's performance with higher field-strength magnets, the authors stated.
BOLD versus MS-325
Blood oxygen level-dependent (BOLD) fMRI is commonly used in neuroimaging, but it requires a heavily T2*-weighted pulse sequence that leads to areas of significant signal intensity loss. In the second study, published in the American Journal of Neuroradiology, Maravilla and his colleagues in Seattle tested MS-325 (Epix Phamaceuticals, Cambridge, MA), which is a long intravenous half-life gadolinium-based contrast agent, in a functional imaging study using T1-weighted pulse sequences.
"This technique (with MS-325) has the advantage of using a T1-weighted pulse sequence, instead of a heavily T2*-weighted sequences, thus potentially reducing areas of susceptibility-related signal intensity loss observed with BOLD fMRI," the group wrote (AJNR, August 2006, Vol. 27:7, pp. 1467-1471).
This study had to be done on adult rats as MS-325 is currently undergoing phase III clinical trials and cannot be tested in humans, Marvilla's group explained. The test group consisted of 18 rats in total that first had BOLD fMRI exams then MS-325 fMRI exams. The control group consisted of eight animals that were imaged with saline as the contrast agent.
All studies were done on a 1.5-tesla whole-body scanner (Horizon EchoSpeed, GE Healthcare, Chalfont St. Giles, U.K.). For the BOLD fMRI tests, a multisection 2D, single-shot, gradient-echo, echo-planar pulse sequence was used. For the MS-325 study, the rats were given 0.1 mmol/kg of the contrast agent, then imaged with a 3D volume acquisition gradient-recalled echo (GRE) pulse sequence. Sensory cortex activation was measured with forepaw electrical stimulation under anesthesia.
According to the results, all the test subjects demonstrated MS-325 fMRI activations in the forepaw sensory cortex. The authors calculated the percentage signal intensity change and found that it was 2.9% in the BOLD measurements and 1.9% in the MS-325 measurements. Also, the volume of activation for BOLD was 9.8 mm³ and 3.2 mm³ for MS-325. There was no significant difference between the two methods for amplitude activation.
The authors stated that the value of MS-325 as a T1-weighted enhancing contrast agent can be attributed to two factors: its two-hour effective half-life and its increased relaxivity, making it more stable during an fMRI exam.
As MS-325 is still under investigation, certain issues still need to be resolved if the agent is to be used in this setting, the authors noted. "The optimal T1 pulse sequence for use in human subjects also remains to be determined. (Also), the optimal dose of MS-325 for fMRI measurements has not been determined," they wrote.
However, the authors pointed out that on a purely technical level, T1-weighted imaging with MS-325 can reduce the susceptibility-related artifacts that are associated with BOLD fMRI.
By Shalmali Pal
AuntMinnie.com staff writer
September 5, 2006
Related Reading
Gray-matter abnormalities seen in autism spectrum disorder, August 22, 2006
Epix, Predix complete merger, August 17, 2006
Pain processing centers in brain show decreased activity in self-mutilators, June 28, 2006
Copyright © 2006 AuntMinnie.com