Rubidium-82 is increasingly being used for cardiac PET imaging, but radiation dose estimates for the exams are based on old -- and conflicting -- data. Researchers from Johns Hopkins University offer updated dose estimates for rubidium-82 procedures in a study published in the October issue of the Journal of Nuclear Medicine.
Rubidium-82 is marketed under the trade name CardioGen-82 by Bracco Diagnostics of Princeton, NJ, and is the most commonly used cardiac PET radiopharmaceutical. The increased attention toward radiation dose from medical imaging procedures has prompted some clinicians to try to gauge the dose delivered in rubidium-82 PET studies, but these attempts have been stymied by wide discrepancies between CardioGen-82's package insert labeling and separate radiation dose estimates generated by the International Commission on Radiological Protection (ICRP).
Researchers from Baltimore-based Johns Hopkins University (JHU) studied rubidium-82 dose in a group of 10 healthy volunteers, and in addition to generating an independent estimate of the radiopharmaceutical's dose, they found that the dose generated during a typical rubidium-92 study is about equivalent to a person's natural background radiation dose for a year. The lead author of the study was Senthamizhchelvan Srinivasan, PhD, in the division of nuclear medicine in JHU's Russell H. Morgan Department of Radiology (JNM, October 2010, Vol. 51:10, pp. 1592-1599).
Inherent limitations
The dose estimates contained in both the CardioGen-82 package insert and the ICRP reports are based on data that are more than 20 years old and contain "inherent limitations," according to senior author Frank Bengel, MD, associate professor of radiology and medicine at JHU.
"There was a huge discrepancy, which was low as published by the manufacturer and very high, on the other hand, as published by ICRP," Bengel said. "Our intention was to use state-of-the-art methodology and look back at the issue to clarify this existing discrepancy and create hard data for the clinical tracer."
Bengel said he and his colleagues also explored the tracer's radiation dose because of the debate about radiation exposure from medical imaging procedures. "We felt that with that discussion -- often a very emotional discussion -- it is important that we create scientific data. It is better to discuss issues based on scientific evidence, rather than emotional feelings about radiation exposure."
The study enrolled 10 healthy volunteers (five men and five women) with no history of cardiac disease. Subjects with evidence of clinical disease, history of organ-removal surgery, or history of substance abuse were excluded.
All 10 volunteers underwent PET/CT imaging (Discovery Rx VCT, GE Healthcare, Chalfont St. Giles, U.K.) in a system that featured lutetium-yttrium oxyorthosilicate (LSO) PET crystals and a 64-slice CT component.
The subjects were imaged at rest in the supine position. Six contiguous single-bed-position PET images were acquired in 2D mode, with 5 mm of overlap. For each imaging position, CT was performed first. PET imaging followed for eight minutes immediately after the start of intravenous infusion of 536 ± 100 MBq of rubidium.
Rubidium half-life
Because rubidium-82 has a very short half-life of only 76 seconds, the researchers repeated the injections at different positions of the body for each of the 10 subjects to accurately measure the biodistribution of all relevant organs.
"We measured the kinetics in each of these organs very accurately, but at only one table position at a time," Bengel said. "Then we moved to the next table position and injected the second dose of rubidium and looked at the organs at that table position."
Each volunteer received six injections of rubidium, but the overall injected dose of the PET agent was not greater than what a usual patient would receive for a rest-stress cardiac myocardial perfusion study.
Bengel noted that the standard dose for a clinical protocol in rest-stress studies is one 40-mCi injection at rest and one 40-mCi injection at stress, for a total dose of 80 mCi or 1,480 MBq. The maximum clinically approved dose for rubidium-82 is 4,240 MBq, while the average total dose per subject in the current study was approximately 3,200 MBq.
In the analysis of the PET/CT images, the researchers found the highest mean absorbed organ doses (mGy/MBq) for the kidneys (5.81), followed by the heart wall (3.86) and lungs (2.96). The results for the three organs were not surprising, according to Bengel.
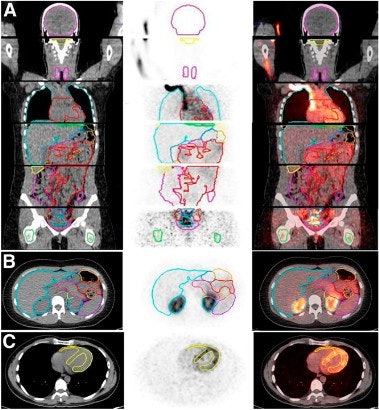 |
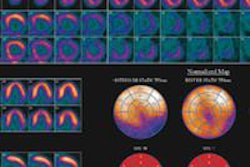
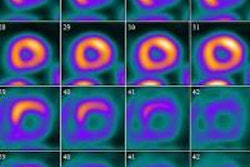
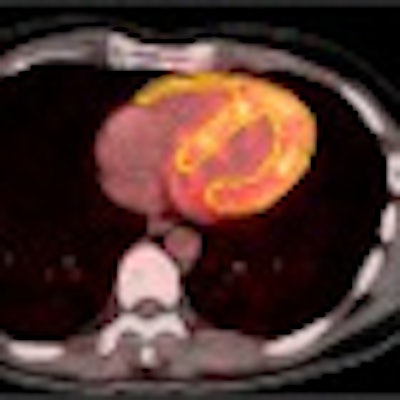
| Image A is a coronal view of six bed positions stacked together (separated by transaxial lines), with color-coded organ contours on CT (left), PET (summed image of all dynamic frames, middle), and PET/CT fusion (right) images. Image B is a transverse view of the upper abdominal region with color-coded organ contours on CT, PET, and PET/CT fusion images. Image C is a transverse view of the heart region, with heart wall contours (yellow) shown on CT, PET (summed image of last 20 frames), and PET/CT fusion images. All images courtesy of the Journal of Nuclear Medicine. |
"We know that rubidium is secreted by the kidneys," he said. "The heart wall gets a high dose, because it is our target organ, so we want a lot of tracer to go to the heart wall in order to get good images. The lungs also get a significant dose, because the high dose of injected tracer has its first pass through the lungs when the tracer is not yet diluted."
Compared with estimates in the ICRP study, the authors noted that CardioGen-82's package insert showed "significantly lower doses to most organs, except for the breast, heart wall, gonads, urinary bladder wall, and uterus." When compared with the package insert, the ICRP report showed higher rubidium dose for the heart wall, lungs, and pancreas.
But the current study found that rubidium-82 delivered a lower dose for the kidneys than either of the two estimates: a threefold difference compared to the ICRP results and 1.5-fold lower than the CardioGEn-82 package insert.
Clinical implications
"We ended up with an effective radiation dose for rubidium-82 which is comparable to other PET myocardial perfusion imaging agents and which is within the range of the average annual natural radiation exposure for subjects in the U.S.," he said. "The effective dose we ended up with made us feel comfortable using this agent in the clinics."
While the study results have prompted no changes in clinical protocols at Johns Hopkins, Bengel said the additional information has "helped us in understanding what the consequences from injecting the radiotracer for our tests are. We can more clearly communicate to the clinician how this would compare to other established radiation exposure numbers."
Bengel and colleagues plan to continue their research to measure dosimetry of rubidium-82 during stress conditions. "In order to calculate the effective dose for a patient for a whole rubidium study, you need to know the dosimetry under rest conditions and under stress conditions," he said. "There might be differences, because blood flow changes during stress conditions and the dosimetry may change, too."
Because this current study only evaluated healthy subjects, Bengel also noted that dosimetry may change in patients with a disease who are referred for PET/CT exams.
By Wayne Forrest
AuntMinnie.com staff writer
October 8, 2010
Related Reading
Cedars-Sinai explores PET's future in nuclear cardiology, April 11, 2008
Rubidium PET/CT shows potential for coronary artery disease diagnosis, March 30, 2007
Cardiac SPECT/CT fusion captures SNM's Image of the Year, June 6, 2006
Rubidium-based PET may finally get its due, February 23, 2006
Copyright © 2010 AuntMinnie.com