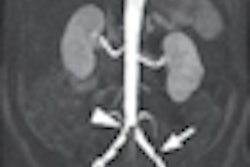
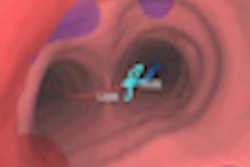
Interventional technology firm Cook Medical has submitted a premarket approval (PMA) application to the U.S. Food and Drug Administration (FDA) for a balloon-expandable stent for renal artery disease.
Targeted at patients suffering from renal artery stenosis, the stent features a low-profile design and does not shorten upon expansion, according to the Bloomington, IN-based vendor. The PMA submission includes data from the REFORM clinical trial, which studied 100 patients at seven investigative sites in the U.S., Cook said.
Related Reading
Cook unveils new NavAlign system, March 16, 2010
Cook adds to PAD line, October 21, 2009
Cook launches antibiotic catheter, May 12, 2009
Cook launches interventional radiology unit, February 2, 2009
Cook opens new Illinois plant, December 12, 2008
Copyright © 2010 AuntMinnie.com