Findings indicative of functionally important coronary artery disease (High risk) [52]:
1- Multiple perfusion defects in more than one coronary artery distribution- reflective of multivessel disease
2- An extensive area of hypoperfusion on stress imaging even if confined to a single vascular distribution (i.e.: proximal LAD pattern)
3- Transient ischemic left ventricular cavity dilatation on stress imaging
4- Post stress gated SPECT ejection fraction of less than 40%
5- Increased lung activity on post stress thallium imaging
6- Increased end-diastolic volume or end-systolic volume (greater than 70 mL) as determined by quantitative SPECT
The perfusion exam report [56]:
The perfusion exam report should contain the following information:
1- Indication for the exam: Diagnosis of coronary artery disease (CAD), delineation of extent and severity of CAD, risk stratification, myocardial viability evaluation, or evaluation of acute chest pain syndromes.
2- Clinical history: Patient symptoms, medications (particularly cardioactive medications), and previous diagnostic tests if relevant.
3- Procedure: The type of stress (exercise versus pharmacologic), the adequacy of the stress (heart rate, blood pressure), symptoms during the exam, and reason for termination. Significant ECG findings (ST segment deviations) can also be included.
4- Findings: The quality of the exam should be reviewed- patient motion, subdiaphragmatic/hepatic activity, soft tissue attenuation. Perfusion defects should be described in terms of their location, size (small, medium, and large), type (reversible, fixed, mixed), and severity (mild, moderate, severe). Summed stress, rest, and difference scores may be reported. Abnormal cavity dilatation (with stress or persistent) and abnormal lung activity should be recorded.
5- Impression: State whether the exam is normal or abnormal- and whether there is single or multivessel disease. Probably normal, probably abnormal, and equivocal should be used as infrequently as possible. If the patient is unable to achieve an adequate level of stress or the images are of inadequate quality, the term "non-diagnostic" should be used to describe the study.
False Positive Stress Perfusion Exam
(Reversible or Fixed Perfusion Defects in the Absence of Coronary Artery Disease at Coronary Angiography)
Reversible perfusion defects have been reported in up to 27% of selected patients with angina and normal findings on coronary angiography [24].
NOTE: The accuracy of myocardial perfusion scintigraphy has previously been evaluated using coronary angiography as the gold standard. Angiography is a silhouette technique with substantial limitations [24]. Recent work with intracoronary sonography indicates that coronary angiography can underestimate the extent and severity of coronary artery disease- particularly in the early stages of atherosclerosis [24]. Reversible perfusion defects on myocardial perfusion scintigraphy associated with normal coronary angiography may actually represent occult, unrecognized atherosclerotic coronary artery disease which is commonly associated with an abnormal vasodilatory capacity [24].
1. Normal variant
a- Diaphragmatic attenuation
Diaphrgamatic attenuation produces a fixed inferior perfusion defect [50]. It can be exaggerated in obesity and with a full stomach. Although gated imaging helps to assess for wall motion, a problems remains because perfusion defects due to subendocardial infarction that are associated with a normal contraction pattern may be falsely attributed to attenuation artifact [50].
Prone positioning can reduce diaphragmatic attenuation and improve the specificity of the exam by producing an anterior shifting of the heart and a lowering of the diaphragm and subdiaphragmatic organs [50]. However, prone imaging may create an anterior or anteroseptal wall perfusion defect and therefore cannot substitute for routine supine imaging (presumably due to sternal or rib attenuation) [50]. Prone imaging can be performed using 15 seconds per projection with 32 or 64 projections for dual or single-headed cameras respectively [50].
Patients with inferior wall defects on supine imaging that are not present on prone imaging have a low risk of subsequent cardiac events (0.7% per year) which is similar to that seen in patients with normal supine exams [50].
b- Breast shadow or breast implant
The breast artifact is most easily appreciated on the rotating tomographic projection or volume rendered images, but can also be seen on planar images. The degree of attenuation varies directly with breast size and tissue density. Classically these defects produce fixed anterior or lateral wall defects, however, any wall can be involved. Remember- body builders with heavily muscled pectoralis muscles have also produced this artifact.
c- Pacemakers
Although pacemakers are implanted above and below the diaphragm, it is unusual for a pacemaker to intrude into the chest image and interfere with image interpretation.
2. Mitral Valve Prolapse
Exercise perfusion defects are uncommon (less than 5%) in patients with mitral valve prolapse, but may be related to exercise induced stimulation of sympathetic activity. This activity produces a tachycardia which will increase the degree of prolapse and consequently stretch the cordae tendinae on the papillary muscles. This stretching may cause ischemia of the papillary muscles and surrounding myocardium. This is only theory, however, and the actual mechanism for these defects is unknown. Since this phenomenon is uncommon, reversible defects in patients with prolapse should still be considered ischemia until proven otherwise. [19]
3. Valvular Heart Disease
a. Mitral Regurgitation
b. Aortic Stenosis:
Coexisting coronary artery disease is found in about 60% of patients with aortic stenosis [53]. Unfortunately, in patients with aortic stenosis, between 40 to 60% of patients without significant coronary lesions demonstrate either fixed or reversible defects. These perfusion defects are probably related to an increased myocardial oxygen demand combined with a decreased perfusion gradient in the coronary arteries associated with a tight aortic stenosis and left ventricular hypertrophy which produces extravascular compression [40,53]. These perfusion abnormalities may improve postoperatively following the relief of stresses on the pressure loaded ventricle. Pharmacologic stress is preferred in patients with severe aortic stenosis because exercise stress may result in hypotension, syncope, or sudden cardiac death [53].
c. Aortic Regurgitation:
The prevalence of angina pectoris has been reported to vary from 50% to 70% of patients with severe aortic stenosis, however, only half of these patients have coronary lesions at angiography [40]. Patients with aortic regurgitation may demonstrate reversible stress apical defects in the absence of coronary artery disease. With eccentric hypertrophy, the perfusion pressure to the "watershed areas" between the coronary vessels decreases to yield apparent perfusion defects. Perfusion defects elsewhere, should be considered indicative of CAD until proven otherwise.
4. Left Bundle Branch Block
In left bundle branch block (LBBB), there is delayed septal activation and relaxation which adversely affects diastolic coronary flow regionally [54]. With exercise, the myocardium experiences decreased diastolic perfusion time which yields decreased radiotracer extraction by the septum relative to the lateral wall [55]. Thus, these patients may have a reversible septal/anteroseptal defect that is most pronounced at high heart rates. This defect should NOT involve the apex- when it does, this favors a diagnosis of ischemia. Pharmacologic stress with dipyridamole or adenosine which do not increase the heart rate significantly (and therefore should not affect diastolic flow) can be useful in these patients. Dobutamine stress, which increases heart rate, can produce this artifact [41].
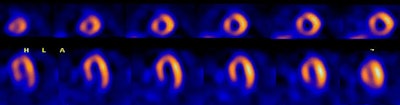
|
Left bundle branch block: The 50 year old male patient shown below underwent Tc-myoview SPECT imaging following exercise stress. The patients ECG revealed a LBBB. SPECT images showed a perfusion defect involving the septal and anterospetal walls that extended to the apical region of the heart. Rest images were not performed due to a high level of concern for coronary artery disease. The patient's coronary angiogram was normal without evidence of a stenosis. |
|
|
5. Idiopathic Subaortic Stenosis
Increased count density in the region of the thickened septum results in an apparent relative decreased activity in the lateral wall which leads to the impression of a fixed defect in this location. Myocardial perfusion imaging may not be able to reliably distinguish CAD in these patients. Nonetheless, the presence of any type of thallium abnormality in these patients is strongly associated with potentially lethal arrhythmias. (Nucl Med Annual 93, p.214)
6. Hypertensive Myocardial Hypertrophy
Despite concentric myocardial hypertrophy in these patients, there is often increased septal radiotracer activity relative to the lateral wall on both stress and rest images. This increased count density leads to a relative decreased count density in the lateral wall, which may be misinterpreted as a fixed lateral wall abnormality.
7. Cardiac contusion
8. Infiltrating myocardial disease
Sarcoid, Amyloid, Neoplasm
9. Cardiomyopathy
a. Dilated Cardiomyopathy
Perfusion defects on rest images can be identified in patients with non-ischemic dilated cardiomyopathies [49] and may be related to partial volume effects associated with thinning of the myocardium with underestimation of the concentration of radioactivity [37]. Another reason for perfusion defects in these patients include areas of myocardial fibrosis [37,49].
b. Hypertrophic Cardiomyopathy:
Ischemia is probably related to an imbalance between oxygen supply in face of the increased myocardial mass, septal perforator arterial compression, abnormalities of intramural arteries, and disturbance of left ventricular relaxation. These patients also demonstrate reversal of the normal lateral-to-septal myocardial count density. Image normalization to the hot septum can create apparent fixed lateral wall perfusion defects.
Apical hypertrophic cardiomyopathy is characterized by asymmetric myocardial hypertrophy of the apex of the left ventricle [51]. Affected patients generally have a benign clinical course with a low risk for sudden cardiac death [51]. On rest imaging, these patients can demonstrate increased apical tracer uptake, while there is decreased apical activity on stress images even in the absence of coronary artery disease [51]. This is likely related to a disproportionate wall-thickness to vascular supply ration (i.e.: decreased flow reserve) [51].
10. Bland-White-Garland Syndrome
(Anomalous origin of the left coronary artery from the pulmonary artery)
This anomaly most commonly results in death during early infancy, but survival into adulthood can occur if collateral coronary flow is sufficient. Patients are generally noted to have an anterior wall ischemic perfusion defect. An inferior/posterior perfusion defect may also be seen secondary to a right coronary artery to left coronary artery to pulmonary artery shunt.
11. Coronary spasm
Coronary artery spasm occurs in angiographically normal coronary artery vessels or superimposed on obstructive coronary artery disease. Patients with Prinzmetal's angina, cocaine overdoses, and collagen vascular diseases like scleroderma are known to have perfusion defects despite normal coronary arteriograms. Provocative testing with ergonovine during cardiac catheterization may demonstrate latent coronary artery spasm.
12. Myocardial bridge
Myocardial bridging is a rare angiographic finding (<1% ). Since the LCX and RCA follow the AV groove, myocardial bridging is almost exclusively seen with the LAD. The coronary artery dips below the epicardium and runs within the myocardium for a short distance. The characteristic angiographic finding is systolic narrowing of the LAD that disappears during diastole. Myocardial bridging that produces a 75% or greater systolic stenosis can demonstrate reversible perfusion defects. Lesser degrees of narrowing are generally not associated with perfusion abnormalities. (Annual 93, p.209)
13. Myocarditis
14. Decreased coronary flow reserve: Syndrome X
Syndrome X is defined as stress-induced anginal pain with a positive stress test for myocardial ischemia, normal findings on coronary angiography, and normal left ventricular function. This is considered a "small vessel disease" syndrome with a reduced coronary vasodilatative reserve of the coronary microcirculation. Its etiology is unclear but may be due to a variable response to exercise and/or pharmacologic stress by the coronary vessels (some dilate more than others) creating an apparent defect. [22]
15. Recent Post-Percutaneous coronary intervention or rotational atherectomy
Post percutaneous coronary intervention scans should be delayed for at least 2 to 4 weeks after the procedure. Scans performed before this time can reveal apparent fixed defects or are falsely positive for ischemia- these findings may be related to transient persistent myocyte dysfunction or spasm/endothelial damage at the site of the procedure. [31,43]
Transient myocardial perfusion defects can frequently be seen following rotational atherectomy [39]. Etiologies include peripheral vessel obstruction by atheromatous debris, release of vasoactive substances with platelet aggregation, and vessel spasm [39].
16. Papillary muscles
Insertions of the anterior and posterior papillary muscles may produce focal hot spots typically seen at the 2 (anterolateral) and 7 o'clock positions on short axis images. This finding may be misinterpreted as ischemia of other walls, particularly the inferior.
A corollary to this is the focal hot spots seen on the short axis images in the 7 and 11 o'clock positions in patients with significant right ventricular hypertrophy.
17. Diaphragmatic Creep
Patients with rapid and deep respiratory excursions following exercise are most likely to demonstrate a gradual upward shift in the position of the heart following exercise which produces an inferior/inferoseptal reversible defect. Waiting 5 -10 minutes prior to imaging generally eliminates this artifact. Cardiac creep is best appreciated on "column mode" sinogram images (or cyclogram) which register "cranocaudal" movement. The tomographic projection images for the column mode sinogram are summed and displayed as if you are facing the patient (coronal images). It is not appreciated on "row mode" sinogram images which register lateral movement. The tomographic projection images for the "row mode" sinogram are summed and displayed as if you were looking down from above the patient (transaxial images). On multiheaded cameras the second detector begins it acquisition at the point just beyond where the first detector completes its movement (between images 32 and 33 for a 64 step acquisition). Diaphragmatic creep will appear as a "jump" at this point in the rotating planar images.
18. Patient Motion
Patient motion is a considerable problem in tomographic imaging which relies on a accurate center of rotation. Motion induced perfusion defects are affected by the type and amount of motion, motion timing, and the number of camera detectors [27]. The magnitude and timing of the motion determine whether an artifact will occur, while the direction and pattern of motion determine the artifacts location [35]. Motion artifacts affect acquisitions with double-headed detectors more than acquisitions with a single-head detector. This assumption is related to the fact that a single occurrence of non-returning motion with a double-headed detector will affect twice as many projections as with a single-headed detector [27]. However, acquisitions with a single-headed detector are twice as long as with double-headed detectors for the same number of collected counts, and therefore, the chance that the patient will move during the acquisition is greater [27]. The heart can develop several characteristic appearances with motion. The "hurricane sign" is seen in the short axis projection- the left ventricle develops the appearance of the National Weather Service symbol for a hurricane and this occurs due to smearing of photon counts in opposing directions around the left ventricle [35]. With motion the heart can also develop a non-rounded, disjointed appearance. Discontinuities of the left ventricular walls, non-anatomic defects, and hot spots are other characteristic patterns of motion artifacts [35]. In general, anteroseptal wall defects are more frequently seen with downward motion, anterolateral wall defects are more frequent with upward motion, and inferior wall defects are usually seen with cardiac motion consisting of multiple bounces [35]. Motion correction programs can be applied to diminish artifacts on SPECT images [35].
Factors which affect the final result of motion include [27,28,35]:
1. Amount of movement: Less than 3mm is not usually visually detectable. Movement less than 6.5mm (1 pixel) is detectable, but is not usually clinically significant. Movement of 13mm or greater (2 pixels) frequently produces quantitative abnormalities. Motion correction programs can improve the exam, but studies with greater than 2 pixels of motion should be repeated [27].
2. Type of movement: Vertical movement (up and down) is usually more significant than lateral movement. Lateral motion, however, is more complex and will have a varying effect on the projection images [27]. For lateral motion, shifting is greatest in the anterior image (where motion is parallel to the detector) and least in the lateral image (where motion is perpendicular to the image) [27]. Patient bouncing usually does not produce significant perfusion abnormalities, whereas non-returning motion will create the largest defects [27]. Rotational motion- in which the body turns about its axis- can also create artifacts [35].
3. Time and duration of movement: Movement at the beginning or end of a study is less likely to result in image artifacts, whereas movement occurring at midaquisition has the worst effect- especially for double-headed detector systems [27]. As a general rule, motion in the projections in which myocardial counts are greater appears to cause larger motion artifacts [27]. This is because the corresponding projections have higher photon counts and contribute more information to the reconstructed SPECT images [35]. Generally, projections in which the camera is closest to the heart will have greater photon counts and will be the most susceptible to motion artifacts [35].
Even considerable motion that affects only one or two frames will unlikely result in a perfusion artifact [35]. Motion that corrupts multiple frames is more likely to produce an artifact [35].
4. Multiheaded camera: The second detector begins it acquisition at the point just beyond where the first detector completes its movement (between images 32 and 33 for a 64 step acquisition). Any movement which occurred during the exam (such as diaphragmatic creep) will appear at this point in the rotating planar images.
Inspection of the projection data in a cine loop format is the best way to detect cardiac motion [35]. Sinograms (for horizontal motion) and cyclograms (for verticle motion) are additional tools for motion detection [35]. These images should appear smooth- any discontinuities in the shape of the spirals are reflective of motion [35]. Although significant motion can introduce image artifacts that may be erroneously interpreted as perfusion defects, it is rather uncommon (approximately 1% of cases) for motion to mask a perfusion defect [35].
18. Acquisition Artifact:
Remember- Perfusion defects occur in the distal tissues subtended by the stenotic artery. Defects involving the proximal septum and/or proximal anterior/anterolateral wall are unlikely to represent ischemia except in patients who have had CABG's. These patients may have high grade occlusions in their native proximal coronary arteries and hemodynamically significant stenoses in their saphenous vein or internal mammary artery grafts.
Abdominal and visceral activity can produce a variety of artifacts. There can be an apparent increase in inferior wall activity due to Compton scatter and or overlap of tracer avid liver or bowel which can mask a perfusion defect [38]. Subdiaphragmatic activity can also produce "intensity artifacts" [38,44]. Filtered back projection reconstruction of acquisition images in the presence of intense subdiaphragmatic activity can lead to suppression of adjacent inferior wall counts. This occurs during reconstruction when cold streak artifacts radiated from the intense area of subdiaphragmatic activity into adjacent structures- similar to bladder activity on SPECT bone imaging of the pelvis (backprojection, particularly with the use of a RAMP filter, creates a large band of pixels with negative counts around hot objects during SPECT reconstruction) [44]. These streaks of decreased activity can radiated into the inferior wall and mask tracer uptake [44,46]. Perfusion defects can also be created due to normalization of the images to "hot" visceral activity.
Cardiac rotation within the chest also affects the apparent tracer distribution. Patients with levorotation have a relative increase in the lateral wall count density, while patients with dextrorotation have a relative increase in the septal wall count density.
The position of the heart in relation to the camera orbit can also produce artifacts when using 180 degree acquisition orbits. Although 180 dgree SPECT images generally display better image contrast, reconstruction images are distorted because of inadequate filtered backprojection [42]. These artifacts of enhanced contrast and geometric distortion are in part caused by the non-uniform and depth-dependent spatial resolution and are further aggravated by the embedded ramp filter in filtered backprojection reconstruction [42]. For 180 degree orbits and filtered backprojection reconstruction, an off-center position of the heart can result in image inhomogeneity with mild perfusion defects in the anteroseptal and inferolateral walls (particularly towards the apex) [42]. The inhomogeneity becomes increasingly more apparent with increasing eccentric cardiac positioning [42]. The explanation for this finding is that when a patients heart is not in the center of the orbit, the gamma heads are at varying distances from the heart during rotation through the orbit [42]. Segments of the left ventricular walls closet to the detector are better resolved and show apparent localized thinning (better resolution), which may appear as mild perfusion defects [42]. These inhomogeneities are not observed with 360 degree acquisition orbits [42]. An interesting consequence of off center cardiac position is that true perfusion defects will be over-estimated on 180 degree acquisitions and slightly underestimated by 360 degree acquisitions [42].
Interpretation schemes for SPECT myocardial perfusion imaging usually use a standardized display in which the stress data set is compared to a resting data set [47]. On most commercial systems, each of these data sets is normalized to the maximum myocardial tracer content (brightest pixel count) in the entire data set prior to interpretation [47]. This permits quantitative comparison of segmental tracer activity whenever the brightest pixel is located in the same myocardial region on the resting and stress images [47]. However, if the area of maximal tracer activity on a resting study becomes relatively ischemic with stress and is no longer the brightest region, the comparison of the two exams loses accuracy [47]. This is because the stress exam will be normalized "upward" to the brightest pixel in a different myocardial segment [47]. Renormalization of regions appearing to increase with stress to their resting level before image interpretation can improve detection of multivessel coronary artery disease [47].
False Negative Thallium Exam
1. Beta blocker/Calcium channel blocker
Unable to obtain adequate stress- inability to reach 85% of the maximal predicted heart rate during exercise may decrease the sensitivity of the exam [31]. The negative chronotropic properties of beta-blockers commonly result in submaximal heart rate response to exercise [31]. However, even in patients on beta-blockers that fail to reach 85% of their maximum predicated heart rate, a normal examination may be associated with an overall good prognosis (annual mortality of 0.6% and 0.4% for major ischemic event in one study) [48].
2. "Balanced" ischemia
Seen in symmetric three vessel disease- there is relatively balanced global hypoperfusion which is not recognized without absolute quantification of regional blood flow [36]. Indirect indicators of three vessel disease with thallium imaging include transient LV dilatation and increased lung activity [36].
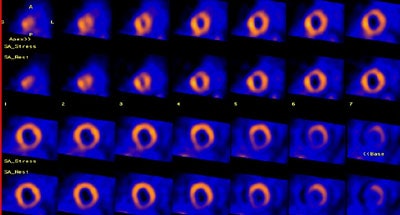
|
Balanced ischemia: The short axis images below are from an adenosine stress exam- stress images are on top and rest images are below. The exam revealed a prominent left ventricle with fixed perfusion defects in the anterior and posterior walls. No significant reversible perfusion defects were identified. At cardiac catheterization the patient was found to have 3-vessel disease. The case below is an example of "balanced" ischemia. |
|
|
3. Insufficient luminal obstruction
4. Inadequate stress
5. Poor technique
Imaging is delayed following stress
REFERENCES:
(1) J Nucl Med 1993; Schulman DS, et al. Right ventricular thallium-201 kinetics in
pulmonary hypertension: relation to right ventricular size and function. 34: 1695-700
(2) New Engl J Med 1993; Zaret BL, Wackers FJ. Nuclear cardiology (1). 329: 775-783
(3) Am J Cardiol 1990; Villanueva FS, et al. Prevalence and correlates of increased lung/heart ratio of thallium-201 during dipyridamole stress imaging for suspected coronary artery disease. 66: 1324-28
(4) Am.J.Cardiol 1990; Lette J, et al. Transient left ventricular cavitary dilation during dipyridamole-thallium imaging as an indicator of severe coronary artery disease. 66:1163-70
(5) J Nucl Med 1994; Iskandrian AS, et al. When is myocardial viability an important clinical issue? 35 (Suppl): 4S-7S
(6) J Nucl Med 1994; Leavitt JI, et al. Demonstration of viable, stunned myocardium with technetium-99m-sestamibi. 35: 1805-07
(7) J Nucl Med 1992; Cuocolo A, et al. Identification of viable myocardium in patients with chronic coronary artery disease: comparison of thallium-201 scintigraphy with reinjection and technetium-99m-methoxyisobutyl isonitrile. 33: 505-511
(8) Circulation 1994; Dilsizian V, et al. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc-sestamibi with thallium reinjection and [18F] fluorodeoxyglucose. 89: 578-87
(9) Radiol Clin North Am 1993; Kiat H, et al. Myocardial perfusion imaging using technetium-99m radiopharmaceuticals. 31: 795-815
(10) J Nucl Med 1994; Altehoefer C, et al. Significance of defect severity in technetium-99m-MIBI SPECT at rest to assess myocardial viability: comparison with fluorine-18-FDG PET. 35: 569-74
(11) J Nucl Med 1995; Maurea S, et al. Enhanced detection of viable myocardium by technetium-99m-MIBI imaging after nitrate administration in chronic coronary artery disease. 36: 1945-52
(12) J Nucl Med 1995; Bisi G, et al. Technetium-99m-sestamibi imaging with nitrate infusion to detect viable hibernating myocardium and predict postrevascularization recovery. 36: 1994-2000
(13) J Nucl Med 1995; Maurea S, et al. Myocardial viability index in chronic coronary artery disease: technetium-99m-methoxy isobutyl isonitrile redistribution. 36: 1953-60
(14) Circulation 1994; 89:578-87
(15) J Nucl Med 1998; Bax JJ, et al. Comparison of Fluorine-18-FDG with rest-redistribution thallium SPECT to delineate viable myocardium and predict functional recovery after revascularization. 39: 1481-1486
(16) J Nucl Med 1995; Ohte N, et al. Clinical significance of reverse redistribution on
24-hour delayed imaging of exercise thallium-201 myocardial SPECT: comparison with
myocardial fluorine-18-FDG-PET imaging and left ventricular wall motion.
36: 86-92
(17) J Nucl Med 1993; Liu P, Burns RJ. Easy come, easy go: time to pause and put thallium reverse redistribution in perspective. 34: 1692-94
(18) J Nucl Med 1995; Soufer R, et al. Relationship between reverse redistribution on planar thallium scintigraphy and regional myocardial viability: a correlative PET study.36: 180-87
(19) Nucl Med Annual 1993; Parmett SR, Ongseng. Myocardial perfusion imaging: Pifalls and pearls. Ed. Freeman LM. Raven Press, NY. 195-221
(20) Radiographics 1999; Jadvar H, et al. SPECT and PET in the evaluation of coronary artery disease. 19: 915-926
(21) AJR 2000; Robinson VJB, et al. Causes of transient dilatation of the left ventricle during myocardial perfusion imaging. 174: 1349-1352
(22) Semin Nucl Med 1997; Kataoka T. False-positive myocardial perfusion scintigraphy in syndrome X. 27 (2): 186-189
(23) J Am Coll Cardiol 1996; Mazzanti M, et al. Identification of severe and extensive coronary artery disease by automatic measurement of transient ischemic dilatation of the left ventricle in dual-isotope myocardial perfusion SPECT. 27: 1612-1620
(24) J Nucl Med 2000; Verna E, at al. "False-positive" myocardial perfusion scintigraphy findings in patients with angiographically normal coronary arteries: Insights from intravascular sonography studies. 41: 1935-1940
(25) J Nucl Med 2001; Bax JJ, et al. Relationship between preoperative vaiability and postoperative improvement in LVEF and heart failure symptoms. 42: 79-86
(26) J Nucl Med 2001; Tawakol A, Gewirtz H. Does CABG improve left ventricular ejection fraction in patients with ischemic cardiomyopathy, and does it matter? 42: 87-90 (No abstract available)
(27) J Nucl Med 2001; Matsumoto N, et al. Quantitative assessment of motion artifacts and validation of a new motion correction program for myocardial perfusion SPECT. 42: 687-694
(28) J Nucl Med 1993; Prigent FM, et al. Effect of motion on thallium-201 SPECT studies: a simulation and clinical study. 34:1845-50
(29) J Am Coll Cardiol 1997 Bax JJ, et al. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Pooled comparison data. 30: 1451-60
(30) Semin Nucl Med 2000; Bas JJ, et al. 18-Fluorodeoxyglucose imaging with positron emission tomography and single photon emission computed tomography: Cardiac applications. 30: 281-298
(31) Semin Nucl Med 1995; Newhouse HK, Wexler JP. Myocardial perfusion imaging for evaluating interventions in coronary artery disease. 25: 15-27
(32) J Nucl Med 2001; Tanaka R, Nakamura T. Time course evaluation of myocardial perfusion after reperfusion therapy by 99mTc-tetrofosmin SPECT in patients with acute myocardial infarction. 42: 1351-1358
(33) J Nucl Med 2001; Harel F, et al. Clinical impact of combination of scatter, attenuation correction, and depth-dependent resolution recovery for 201Tl studies. 42: 1451-56
(34) Radiology 2001; Oshinski JN, et al. Quantitative prediction of improvement in cardiac function after revascularization with MR imaging and modeling: Initial results. 221: 515-522
(35) J Nucl Cardiol 2001; Fitzgerald J, Danias PG. Effect of motion on cardiac SPECT imaging: recognition and motion correction. 8: 701-706
(36) J Nucl Med 2002; Yamagishi H, et al. Incremental value of left ventriuclar ejection fraction for detection of multivessel coronary artery disease in exercise 201Tl gated myocardial perfusion imaging. 43: 131-139
(37) J Nucl Med 2002; Hassan N, et al. 201Tl SPECT abnormalities, documented at rest in dilated cardiomyopathy, are related to a lower than normal myocardial thickness but not to an excess in myocardial wall stress. 43: 451-457
(38) J Nucl Cardiol 2002; Samady H, et al. Pharmacologic stress perfusion imaging with adenosine: Role of simultaneous low-level treadmill exercise. 9: 188-196
(39) J Nucl Cardiol 2002; Koch KC, et al. Mechanisms of myocardial hypoperfusion during rotational atherectomy of de novo artery lesions and stenosed coronary stents: insights from serial myocardial scintigraphy. 9: 304-311
(40) J Nucl Cardiol 2002; L'Abbate A, et al. Myocardial perfusion and coronary microcirculation: from pathophysiology to clinical application. 9: 328-337
(41) J Nucl Med 1994; Tighe DA, et al. False-positive reversible perfusion defect during dobutamine-thallium imaging in left bundle branch block. 35:1989-91
(42) J Nucl Med 2002; Liu YH, et al. Differential effect of 180 degrees and 360 degrees acquisition orbits on the accuracy of SPECT imaging: quantitative evaluation in phantoms. 43: 1115-1124
(43) J Nucl Cardiol 2002; Angiolillo DJ, Giordano A. Role of myocardial perfusion imaging after coronary revascularization in symptom-free patients: are low-risk patients really low? 9: 550-553
(44) J Nucl Cardiol 2002 Funahashi M, et al. Application of pixel truncation to reduce intensity artifacts in myocardial SPECT imaging with Tc-99m tetrofosmin. 9: 622-31
(45) J Nucl Cardiol 2003; De Lorenzo A, et al. Use of atropine in patients with submaximal heart rate during exercise myocardial perfusion SPECT. 10: 51-55
(46) J Nucl Cardiol 2003; Boz A, et al. The effects of solid food in prevention of intestinal activity in tc-99m tetrofosmin myocardial perfusion scintigraphy. 10: 161-167
(47) J Nucl Cardiol 2003; Williams KA, et al. Correct spatial normalization of myocardial perfusion SPECT improves detection of multivessel coronary artery disease. 10: 353-360
(48) J Nucl Cardiol 2003; Marie PY, et al. Residual exercise SPECT ischemia on treatment is a main determinant of outcome in patients with coronary artery disease treated medically at long-term with beta-blockers. 10: 361-368
(49) J Nucl Cardiol 2003; Wu YW, et al. Tl-201 myocardial SPECT in differentiation of ischemic from non-ischemic dilated cardiomyopathy in patients with left ventricular dysfunction. 10: 369-374
(50) J Nucl Med 2003; Hayes SW, et al. Prognostic implications of combined prone and supine acquisitions in patients with equivocol or abnormal supine myocardial perfusion SPECT. 44: 1633-1640
(51) J Nucl Cardiol 2003; Ward RP, et al. Resting "solar polar" map pattern and reduced apical flow reserve: characteristics of apical hypertrophic cardiomyopathy on SPECT myocardial perfusion imaging. 10: 506-512
(52) J Nucl Cardiol 2003; Beller GA. Clinical value of myocardial perfusion imaging in coronary artery disease. 10: 529-542
(53) J Nucl Cardiol 2004; Patsilinakos SP, et al. Adenosine stress myocardial perfusion tomographic imaging in patients with significant aortic stenosis. 11: 20-25
(54) J Nucl Cardiol 2004; Kasai T, et al. Decreased septal thickening in patients with left bundle branch block. 11: 32-37
(55) J Nucl Cardiol 2004; Hansen CL. The conundrum of left bundle branch block. 11: 90-92
(56) J Nucl Cardiol 2003; Hendel RC, et al. American society of nuclear cardiology consensus statement: reporting of radionuclide myocardial perfusion imaging studies. 10: 705-708