Computer-aided detection schemes are getting more attention in virtual colonoscopy -- an encouraging sign due to CAD's potential to solve a number of VC's most persistent challenges.
A reliable CAD system could potentially help separate polyps from fecal matter, leading to easier bowel prep and thus better patient acceptance. And CAD could potentially speed up the search for polyps in massive CT datasets, while helping to reduce the number of missed lesions.
Like the CAD techniques developed for other anatomic areas, virtual colonoscopy CAD works by analyzing CT attenuation to pick out potential polyps. For example, a CAD technique developed by Dr. Hiroyuki Yoshida and colleagues at the University of Chicago assigns shape index values to attenuation patterns to indicate ruts, cups, saddles, ridges, and caps that represent adenomatous colorectal polyps on CT.
However, CAD schemes tend to produce large numbers of false-positive results, which must then be analyzed individually by the radiologist, or processed by another software application that eliminates some of the inaccurate results.
In a pilot study at the University of Chicago, one CAD technique detected all 11 biopsy-proven polyps in 43 virtual colonoscopy cases, with 2.5 false-positive (FP) findings per case. Yet 20% of the false-positive results were attributable to extracolonic components (EC) from the small bowel and stomach in the CT dataset (Radiology, February 2002, Vol. 222:2, pp. 327-336).
Writing in the Journal of Computer Assisted Tomography, Dr. Janne Näppi, Dr. Abraham Dachman, Dr. Peter MacEaneany, and Dr. Hiroyuki Yoshida evaluated a colon segmentation technique designed to reduce the number of false positives detected by CAD (July-August 2002, Vol. 26:4, pp. 493-504).
"Because these are extracolonic components that should not have been included in the segmented colon ... we could improve the performance of our CAD scheme simply by improving the colonic segmentation technique," wrote the authors, who are also on the team that developed the CAD system.
The group sought to improve on earlier colon segmentation methods that rely on a volume-growing technique and manually placed seed points. Such techniques have been slow, or failed to remove significant amounts of extracolonic image data. Extracolonic data was also the principal shortcoming of an earlier version of the group's segmentation technique, known as anatomy-oriented colon segmentation (AOCS), also reported in the Journal of Computer Assisted Tomography (July-August 2001, Vol. 25, pp. 629-638).
The group's latest knowledge-guided colon segmentation system (KGS) consists of three steps, including anatomy-based extraction (ABE), a segmentation system designed to improve on the AOCS system. ABE extracts most of the visible colonic walls, but may contain extracolonic components, such as small bowel, that adhere to the colonic wall. The second step, colon-based analysis (CBA), is designed to create an alternative segmentation without the extracolonic components. The third step compares the results of both ABE and CBA by highlighting the areas that intersect.
The segmentation technique digitally removes the "outer air" regions surrounding the body, the bones, and the lung base, and segments the remaining colon by thresholding the range of the CT and gradient-magnitude values corresponding to the colonic wall, the authors wrote.
"The ABE adds three new steps to the outer-air segmentation process. The first step inverts the outer-air segmentation of AOCS, and it removes all but the largest resulting connected component, which is the body region," the authors wrote. "The second step fills the air gaps at the surface of the segmented outer-air by volume growing. Thirdly, after the osseous structures have been identified, we remove the body region dorsal to the spine."
The next step minimizes the amount of colon wall that is excluded from the segmented colon by defining the largest connected component of the segmented colon obtained after thresholding for the colonic walls, then adding larger components.
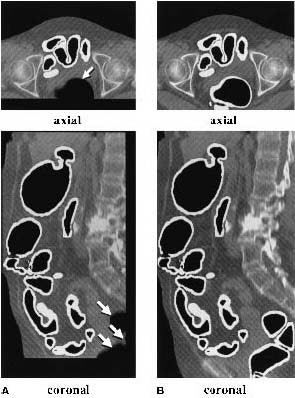 |
| (A) The original anatomy-oriented colon segmentation (white region) could exclude small disconnected regions of the colon such as the rectum. (B) The improved anatomy-based extraction includes the rectum correctly in the segmented region. Note that in this example, the improved ABE produces a larger volume of interest to cover the entire colon. Image and caption reproduced courtesy of the Journal of Computer Assisted Tomography. |
Colon-based analysis (CBA) uses the ABE-segmented colon to guide volume-growing within the colonic lumen, excluding extracolonic components because they are generally not connected to the colonic lumen.
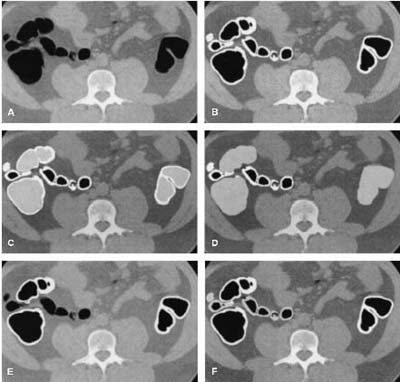 |
| Axial view of (A) original slice; (B) segmented colon obtained by anatomy-based extraction (white region); (C) segmentation of colonic lumen by volume growing (light gray); (D) expansion of the segmented colonic lumen; (E) intersection of the regions segmented by ABE and colon-based analysis (CBA) (white); (F) simultaneous representation of the final knowledge-guided colon segmentation (white), and the regions removed from the ABE (light gray). Image and caption reproduced courtesy of the Journal of Computer Assisted Tomography. |
However, CBA can be complicated by collapsed regions of the colon, which can stop the volume-growing process. When this occurs, CBA partially compensates for the problem by automatically placing multiple volume-growing seeds. The system then evaluates five conditions to determine if CBA segmentation is complete, and compares ABE and CBA results to complete the segmentation.
Then the CAD process begins. Voxel regions with polyp-like characteristics are extracted from the segmented colonic walls by use of hysteresis thresholding of shape index and degree of curvature. The CAD scheme relies on fuzzy c-means clustering, followed by quadratic discriminant analysis to classify polyp candidates as either true positives or false-positives.
The system was tested in a study of 44 subjects, who underwent both conventional and virtual colonoscopy for evaluation by the CAD scheme. Images were acquired using a 9800 spiral CT scanner (GE Medical Systems, Waukesha, WI).
Collimation was 5 mm, pitch 1.5-1.7, and reconstruction intervals ranged from 1.5 to 2.5 mm. Patients were imaged in both supine and prone positions, and each of 88 datasets contained 150-300 CT slices (512 x 512 image matrix) covering the entire colon and rectum. There were 12 colonoscopically proven polyps in 11 patients, including 9 polyps 5-10 mm in diameter, and polyps of 12, 25, and 30 mm, according to the authors.
The CT data were interpolated linearly in the axial direction to yield isotropic voxels, which are needed for automated polyp detection, the authors wrote. The segmentation was evaluated both subjectively and objectively. Four radiologists used a graphical visualization tool to evaluate the segmentation, rating:
- Colon coverage by ABE.
- Colon coverage by KGS.
- Percentage of extracolonic structures in the segmented region.
- Percentage of extracolonic structures in the removed region.
Results
The 44 cases were used to evaluate the knowledge-guided segmentation (KGS) system as a whole, and compare its results to ABE. ABE was expected to show an improvement over the previous AOCS technique, which had included more than 95% of the colon walls in 85% of the segmentations performed.
Average coverage of ABE was 99% of the colon, and average KGS coverage was 98%. The percentage of the region removed from the ABE varied between 1% and 46%.
The average reduction in the segmented region was 15%, and only 1% of the region removed by the KGS technique was associated with the colonic wall, the authors wrote. Before performing CAD, the team visually confirmed that each polyp found in optical colonoscopy was visualized in both the ABE and KGS colon segmentations. In a case-based analysis (using both prone and supine datasets) the colon segmentation reduced the number of false positives by 15%-20% at 100% sensitivity.
"If the colon is fully distended and the colonic lumen does not connect directly to extracolonic structures, the KGS technique can produce a complete and nonredundant segmentation of the colonic walls," the authors wrote. "Even though the ... patients in this study had been subjected to intravenous glucagon and colonic cleansing and distension, several cases contained collapsed regions which caused suboptimal segmentation. Therefore it is necessary to increase the robustness of the KGS technique by more detailed modeling of the expected anatomy of the colon."
Also, ABE sometimes removed parts of the colon wall, but these areas could potentially be recovered by using the CBA-segmented colonic wall, they added.
All 12 colonoscopically proven polyps were contained in both the ABE and KGS segmentations. Further improvement of colon segmentation could probably improve the dataset-based specificity of the CAD scheme by about 10%-15%, since the team found that a corresponding percentage of the region segmented by KGS is still composed of stomach and small bowel.
"Compared with our previously used AOCS technique, the new technique reduces the amount of extracolonic components by 50%, with 10%-15% of the resulting segmentation still composed of extracolonic components," they wrote. The results suggest that further adjustment of the technique may reduce the dataset-based FP rate of the CAD scheme by an additional 10%.
By Eric BarnesAuntMinnie.com staff writer
October 18, 2002
Related Reading
Virtual colonoscopy: 2-D vs. 3-D primary read, June 3, 2002
Prepless virtual colonoscopy shows early promise, June 3, 2002
New CAD technique improves CT colonography, December 21, 2001
Copyright © 2002 AuntMinnie.com