Two-year cosmetic outcomes were good to excellent for nearly 800 women who had accelerated partial-breast irradiation (APBI) treatment for early-stage breast cancer using a multichannel single-entry brachytherapy device called a strut-adjusted volume implant (SAVI).
The SAVI applicator (Cianna Medical) is a type of APBI brachytherapy device that can be expanded to conform to the site of a tumor cavity and a patient's specific anatomy. Radiation dose can be delivered at different strengths using multiple catheters, even when the tumor cavity is located near the skin surface or close to the chest wall. Since December 2010, 1,010 women have been treated with the SAVI applicator.
The SAVI device first came on the market in 2008, and clinical outcomes with the applicator were discussed in a presentation at the National Consortium of Breast Centers (NCBC) meeting in Las Vegas in March, where researchers reported the cosmetic results of 798 women who had lumpectomies followed by adjuvant SAVI treatment. This was the first time that cosmesis outcomes for a large number of patients who had been treated with the SAVI applicator were reported.
Members of the SAVI Collaborative Research Group, which includes 15 U.S. cancer treatment centers using the SAVI applicator, began enrolling patients in a database in April 2010. The database now includes more than 1,000 patients, representing the second largest retrospective database of patients treated with any form of APBI.
Overall, the cosmetic appearance of breasts treated with SAVI was highly favorable, even for women who had radiation delivered near the surface of the skin, according to presenter Dr. Robert Hong, medical director of radiation oncology at Virginia Hospital Center in Arlington. He presented data on patient outcomes at two years, one year, and six months following treatment.
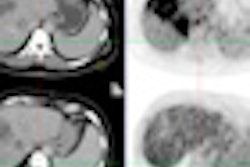
Out of 155 women who had undergone treatment two years ago, 91.6% had good to excellent outcomes. Almost one-third of this group of patients had their breast tumor located close to the skin, with skin spacing less than 7 mm at the time of treatment.
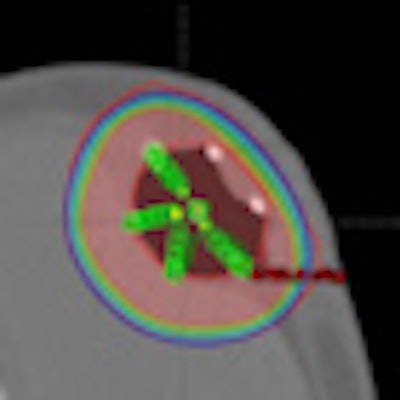
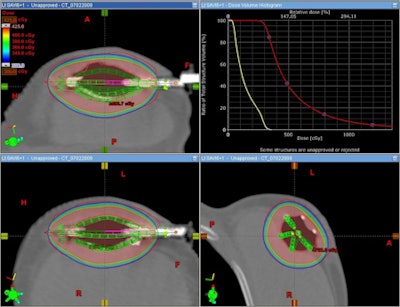 |
| SAVI 6-1 mini plan in a patient with less than 7-mm skin spacing. Image courtesy of SAVI Collaborative Research Group. |
All but 6.3% of the 355 women who had reached the one-year milestone reported good to excellent cosmetic outcomes, and all but 2.4% of 288 women reported good to excellent cosmesis six months following treatment.
Of the 13 women who had fair or poor cosmesis two years following treatment, seven had skin spacing of 7 mm or less, seven had experienced seroma, and one had experienced a breast-related wound infection.
"We looked at cosmetic outcomes in part because it is important to our patients," he said. "This data points toward an additional quality-of-life benefit from the therapy."
Dosimetric characteristics
The SAVI Collaborative Research Group also explained the dosimetric characteristics of strut-based APBI devices during a poster presentation at the American College of Radiation Oncology (ACRO) annual meeting, held two weeks earlier in Fort Myers, FL.
Data were presented on 817 patients treated at 12 of the group's cancer centers. Dosimetric measurements were made for four sizes of applicators, each having six, eight, or 10 peripheral struts that expand around a single lumen.
The study data showed that the maximum dose to the skin rarely exceeded 100% of the prescription dose, said Serban Morcovescu, a medical physicist at Texas Oncology in Denton. For 687 patients, the maximum skin dose was 85% of the prescribed dose, with a range of 57% to 113%.
He noted that skin spacing varied widely, with 460 reported values ranging from 0.5 mm to 76.4 mm. Two-thirds of the patients had skin spacing greater than 7.0 mm, and slightly less than 10% had skin spacing of 3.0 mm or less.
The minimum skin bridge for the 460 patients averaged 12.1 mm, but each of the devices had been used in a total of 44 patients who had a skin bridge of 3.0 mm or less. Chest wall bridge measurements were also reported for 317 patients. They ranged from 0.0 mm to 93.5 mm. For each applicator size, the maximum dose to the chest wall was 75%, with a range of 41% to 109%.
These two reports were the first in a series relating to patient outcomes, disease control, and overall quality of treatment plans with the SAVI applicator for APBI treatment. The series is being presented this year at major medical conferences.