By using gold nanoparticles (AuNPs) in conjunction with conventional megavoltage x-rays, researchers at Brigham and Women's Hospital, Dana-Farber Cancer Institute, and Harvard Medical School in Boston are predicting that the tumor vasculature will receive a significant enhancement in local dose. Not only could this dose boost promote vascular shutdown in tumors, the hope is that it will suppress metastases and improve patient outcomes.
"Based on the promising results of our theoretical calculations, we believe that the use of AuNPs represents enormous possibilities for improving radiation therapy," assistant professor Ross Berbeco, PhD, from Harvard's department of radiation oncology told medicalphysicsweb. "Our goal is to develop an agent that can be used clinically in combination with conventional radiotherapy beams to provide an additional boost of destructive energy to an important piece of the tumor architecture."
Targeted therapy
One as yet unanswered question in radiotherapy is the role that tumor vasculature and, in particular, endothelial cells play in the success of therapy. Over the past few years, nanoparticles have gained much interest as either platforms to carry tumoricidal drugs or as agents themselves for enhancing therapy. In this study -- published online December 15, 2010, in the International Journal of Radiation Oncology, Biology, Physics -- the researchers exploit the fact that nanoparticles preferentially accumulate in the tumor vasculature and are essentially dormant until they are bombarded by an x-ray source.
In the presence of low-energy x-rays of around 100 keV, the AuNPs emit photoelectrons that travel a very short distance before depositing their energy in the nearest endothelial cell. "Others have dismissed the dose enhancement that could be initiated by a 6-MV linac photon energy spectrum," commented Berbeco. "We realized that the dose enhancement is sizable at a short distance from the nanoparticles and that the local radiation boost is actually quite substantial. Our method is a doubly targeted therapy. The nanoparticles target the tumor vasculature while the megavoltage radiation targets the tumor."
Modeling the interactions
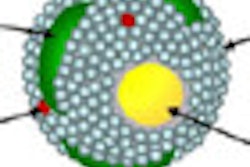
Berbeco and colleagues modeled a tumor vascular endothelial cell as a thin slab measuring 2 x 10 x 10 µm, where a circle of seven cells forms an idealized segment surrounding a blood vessel. Nanoparticles with a diameter of 100 nm are then modeled in a random distribution along the interior endothelial cell wall.
The team presents its results using a value called the "endothelial dose enhancement factor" (EDEF) -- a ratio of the dose absorbed by the endothelial cells in the presence of AuNPs divided by the absorbed dose without AuNPs. The overall EDEF depends on the local concentration of nanoparticles.
According to Berbeco, the majority of the dose enhancement originates from the low-energy (100 keV) portion of the linac x-ray spectrum. Taking into account the probability of a 100 keV photon generating a photoelectron in a 100 nm AuNP, the extra dose deposited in an adjacent endothelial cell is approximately 3.7 mGy per photoelectron per nanoparticle. At a depth of 20 cm, this equates to an EDEF of 1.7 (a 70% dose increase) for a local intravascular AuNP concentration of 30 mg/g.
"We were surprised to find that so much endothelial dose enhancement was possible for a conventional 6-MV linac beam," commented Berbeco. "This opens up some exciting possibilities for new therapies."
Putting theory into practice
The team is now setting up in vitro and in vivo studies to test its hypothesis and quantify the extent to which gold nanoparticles can cause tumor endothelial cell damage. "In vivo studies will be needed to quantify the amount of gold that can be safely sequestered in the tumor vasculature," said Berbeco. "It is also worth noting that for a flattening filter free beam, the proportion of low-energy photons will be substantially increased which should lead to a further enhancement of the dose."
By Jacqueline Hewett
Medicalphysicsweb contributing writer
February 16, 2011
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.