Researchers at Carnegie Mellon University have developed an MRI-based technique for noninvasively following and studying neural stem cells in vivo, potentially leading to new treatments for brain injuries caused by trauma, stroke, Parkinson's disease, and other neurological disorders.
The study, published online September 12 in the journal NeuroImage, is authored by associate professor of biological sciences Eric Aherns, PhD, and biological sciences postdoctoral student Bistra Iordanova.
Neural stem cells are created in the subventricular zone of the brain. In time, these cells, known as neuroblasts, travel to other areas of the brain where they mature into functioning neurons.
If researchers can better understand the molecular migratory signals that guide neuroblasts, these cells may be redirected to areas of the brain harmed by stroke or traumatic injury, thus repairing the brain, Ahrens said.
Studying cells in a living brain, however, is not an easy task. Common forms of in vivo cell imaging, such as fluorescence and bioluminescence, rely on light to produce images, making these technologies unsuitable for viewing neuroblasts deep beneath the skull and layers of opaque tissue, according to the authors.
Until now, scientists have only studied neuronal stem cells by viewing brain slices through a microscope. The MRI technology developed by the Carnegie Mellon researchers has circumvented this obstacle.
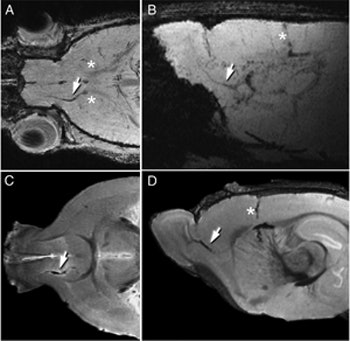 |
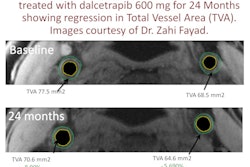
| MRI can be used to follow the migration of neuroblasts tagged with a ferritin-based reporter. Tagged cells are visible in each image (white arrows). Images courtesy of Carnegie Mellon University. |
Several years ago, Ahrens and colleagues developed a way to prompt cells to produce their own contrast agent, so the cells could be imaged with MRI. Using a viral vector, Ahrens incorporated the gene that produces the naturally occurring metalloprotein ferritin into living cells. Ferritin is present in all biological cells and harvests and stores naturally occurring iron.
When cells tagged with ferritin begin to produce increased amounts of the protein, they accumulate additional iron, turning themselves into nanomagnets. This process disrupts the magnetic field surrounding the tagged cells, changing the signal given off by adjacent water molecules. This change appears as dark spots on an MR image, indicating the cells' presence.
Since then, Ahrens' team has improved the process, developing an engineered form of ferritin that is a more effective MRI reporter than naturally occurring ferritin.
In the current study, Ahrens and Iordanova employed the same technique as in the initial study, this time tagging the neuroblasts with the engineered ferritin. They incorporated the DNA sequence for the engineered metalloprotein into an adenovirus vector, which was then injected into the subventricular zone of a rat brain.
The adenovirus infected the neural stem cells, giving the cells the genetic instructions to begin producing the ferritin reporter. Iordanova then imaged the brain with MRI and found that she was able to follow the neuroblasts in real-time as they traveled toward the olfactory bulb and ultimately formed new inhibitory neurons. These results mirrored what had been observed in histology studies.
Ahrens and colleagues plan to continue developing the technology to help researchers better understand neuronal stem cells and how neurons regenerate.
They also plan to use the reporters to improve clinical trials of cell-based therapies. By incorporating the reporter into the cells before implantation, they could determine where the cells go days, weeks, and even months later, and whether they have grafted to the correct cells.
The U.S. National Science Foundation and the National Institutes of Health funded the research.