(Booth 922) Siemens Healthcare of Malvern, PA, will emphasize MRI oncology applications in its RSNA booth at this year's RSNA show.
syngo Grace is pending 510(k) clearance by the U.S. Food and Drug Administration.
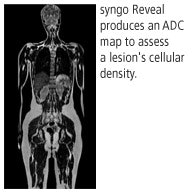
syngo Reveal is designed to differentiate between benign and malignant lesions. An apparent diffusion coefficient (ADC) map is processed automatically at the end of the scan to provide additional information on cellular density of a lesion. A low value in the ADC map would tend to indicate malignancy.
Siemens also will introduce its Prostate MRS@3TMRI scanner as a work-in-progress for prostate spectroscopy.




















