Dutch researchers have found that FDG-PET/CT can identify early response to preoperative chemotherapy with erlotinib in most patients with non-small cell lung cancer (NSCLC), potentially avoiding unnecessary toxicity from ineffective treatment, according to a study in the September issue of the Journal of Nuclear Medicine.
Though the study was relatively small, the researchers from four hospitals in the Netherlands concluded that the results are promising and consistent with the results of preclinical studies. The lead author was Tjeerd Aukema, MD, from the department of nuclear medicine at Haga Hospital in the Hague (JNM, September 2010, Vol. 51:9, pp. 1344-1348).
Erlotinib, an epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor, has been used over the past several years to treat non-small cell lung cancer. Previous research has found that the molecular-targeted agent can prompt early responses to treatment in certain patients with NSCLC and prolong survival when administered as a second-line treatment in advanced cases.
NSCLC survival
The survival rate for NSCLC patients has not improved greatly over the past few years, the authors wrote, and the number of patients presenting with stage IV disease has increased. They noted that the advance is likely the result of better staging, as the metastatic disease is identified prior to clinical symptoms, in part, through FDG-PET's ability to determine oncologic staging.
Therefore, they hypothesized that FDG-PET/CT may be a "valuable clinical predictor" for early response to preoperative erlotinib treatment.
The study enrolled eight men and 15 women (mean age, 63 years) from October 2006 to March 2009. All 23 patients were diagnosed with stage I to stage III non-small cell lung cancer and were eligible for surgical resection. They also were part of an ongoing phase II trial at the four Dutch hospitals of the researchers.
Staging procedures included contrast-enhanced CT and PET/CT scans. With mediastinal metabolic uptake or nodes greater than 1 cm in the shortest diameter, additional endoscopic ultrasound-guided fine-needle aspiration cytology or mediastinoscopy was used.
Erlotinib treatment
The patients received preoperative erlotinib (150 mg) once daily for three weeks, with FDG-PET/CT scans performed before and after erlotinib administration to obtain both baseline FDG-PET/CT and follow-up results. The median time between the start of erlotinib therapy and follow-up FDG-PET/CT imaging was six days.
The baseline FDG-PET/CT scans (Gemini TF, Philips Healthcare, Andover, MA) were obtained during routine staging in all patients with FDG doses of 180 to 240 MBq. Low-dose CT images also were acquired without intravenous contrast. The median time between these two scans was 21 days.
The researchers measured changes in tumor FDG uptake during treatment by prospectively assessing standardized uptake values (SUVs). Patients with a decrease in SUV of 25% or more after one week were classified as "metabolic responders." Their metabolic response was compared with the pathologic response, which was obtained through histopathologic examination of resected specimens.
SUV comparisons
Aukema and colleagues found the median SUVmax at baseline FDG-PET/CT to be 11.0, while the median SUVmax after one week of erlotinib therapy was 9.3. In addition, six (26%) of the 23 cases had a partial response within one week, 16 patients (70%) had stable disease, and one patient (4%) had progressive disease.
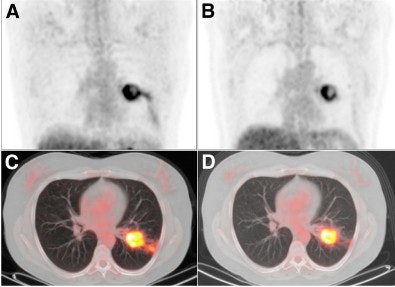 |
| Coronal FDG-PET images show a patient with carcinoma in the left lung. Maximum intensity projection (MIP) images before (A) treatment with erlotinib show a reduction in uptake (ΔSUVmax, -57%) and increase in necrosis of primary tumor after seven days (B) of treatment with erlotinib. The results are also noted in transverse PET/CT fusion images before (C) and after seven days (D) of erlotinib. After erlotinib treatment, the patient was operated on; the resected specimen contained 80% necrosis. Image courtesy of the Journal of Nuclear Medicine. |
FDG-PET/CT also revealed a median 40% necrosis in the resection specimens of treated patients. Among patients classified as metabolic responders, the median percentage necrosis was 70%, compared with a median 40% necrosis among metabolic nonresponders.
The prospective study suggests that during the course of preoperative erlotinib treatment for NSCLC patients, FDG-PET/CT "can identify response in most patients," the authors concluded. "Even though our study was relatively small, the results are promising and consistent with the results of preclinical studies."
They recommended that additional data from larger groups of patients are needed to definitively determine "the optimal timing of response evaluation and the relevance of cutoff values of response parameters."
By Wayne Forrest
AuntMinnie.com staff writer
September 17, 2010
Related Reading
FDG-PET before RT can modify planned NSCLC treatment, August 11, 2010
PET/CT beats CT in NSCLC staging, but both have limitations, July 2, 2010
ASTRO: Use PET for NSCLC judiciously, November 3, 2009
FDG-PET/CT aids in radiation therapy planning, March 5, 2008
PET does not predict survival in advanced lung cancer, November 29, 2007
Copyright © 2010 AuntMinnie.com