Positron has had its ups and downs, to say the least, over the past decade, but the PET device developer thinks it now has a strategy and product to become a more prominent player in the nuclear medicine market.
Fishers, IN-based Positron is banking on the appeal of Attrius, its PET-only device designed for cardiac imaging at community and rural hospitals as well as cardiology practices.
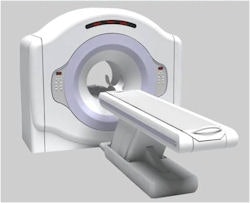 |
| Positron's Attrius cardiac PET scanner. Image courtesy of Positron. |
In 2003, the pair was recruited by a community hospital in Niagara County, NY, which had one of the worst coronary disease death rates per county in the U.S. Like many community hospitals in the country, the facility had a difficult time competing against larger tertiary care centers, Oliverio said, and didn't have a unique cardiovascular offering to stop the migration of patients to other facilities, or to attract patients from other hospitals.
With the help of cardiac PET, the facility was successful in reducing the heart attack and cardiac death rate in the county, as well as the number of angiograms that were being performed, by providing a more accurate diagnosis of cardiac disease through PET, Oliverio said.
Bucking the PET/CT wave
Oliverio acknowledges that the strategy bucks the popularity of PET/CT systems among healthcare facilities. A recent report by market research firm IMV Medical Information Division of Des Plaines, IL, found that no PET-only scanners were installed in the U.S. in 2008; PET-only devices made up 3% of the total installed in 2007 and 60% of systems installed in 2001.
"Everything is going PET/CT, but I didn't see the value in having a combination PET/CT device for cardiovascular use," he said. "There is the benefit of a CT angiogram with PET perfusion, but I am not buying the argument that it needs to be in the same setting. I understand with oncology there is a great benefit in having the patient on the same [PET/CT] table, but with cardiovascular imaging, I didn't see the economic benefit or the clinical benefit."
So, with the expiration of some patents, Positron set out to design and manufacture a less expensive PET-only camera for the community hospital and cardiologist office market. The first key step was to partner with Neusoft Medical Systems of Shenyang, China, starting in January 2006, to begin the manufacture of a PET-only system.
Positron submitted its 510(k) clearance application for Attrius to the U.S. Food and Drug Administration (FDA) in January. Once cleared, the company could begin marketing the device in the U.S. this year.
Oliverio said the Neusoft Positron Medical Systems joint venture could produce as many as 50 PET-only systems per month, if necessary. Positron has exclusive marketing rights for Attrius in the U.S., while Neusoft has exclusivity in China. The two companies share marketing rights in the rest of the world.
Price point
Oliverio did not offer a specific price for Attrius, other than to say it is "priced significantly less than cardiologists' choices for new PET/CT scanners."
The device offers a coronary disease quantitative software package written by cardiac PET pioneer Dr. K. Lance Gould from the University of Texas Medical School at Houston. In addition, Positron plans to offer ejection fraction analysis and CT angiography fusion with PET data through third-party software.
Oliverio is optimistic that Attrius will be well received by healthcare providers looking for a cardiology niche. "We know that the cardiac PET market is expanding, primarily because we see large and mid-sized pharmaceutical companies trying to develop a single-dose F-18-based cardiac perfusion agent," he said. "They wouldn't be developing it if they didn't feel the market wasn't going to flourish."
Positron also has been in contact with Bracco Diagnostics of Princeton, NJ, which markets the PET radiopharmaceutical CardioGen-82 (rubidium Rb 82 generator), the only generator-based PET cardiac perfusion agent approved by the FDA and reimbursed for coronary artery disease evaluation.
"We have been in close contact with them and they have reassured us that there is a significant demand for [a PET-only cardiac system]," Oliverio said.
PET's efficacy
Previous research also would appear to support the efficacy of cardiac PET. Researchers from the State University of New York at Buffalo, led by Merhige, found that the use of PET myocardial perfusion imaging with rubidium-82 in patients with intermediate coronary artery disease resulted in a greater than 50% reduction in invasive coronary arteriography and coronary artery bypass graft surgery.
Merhige, who also serves as director of the Heart Center of Niagara in Niagara Falls, NY, and colleagues also wrote that there was a 30% cost savings by avoiding additional procedures, and patients experienced "excellent clinical outcomes at one year compared with SPECT" (Journal of Nuclear Medicine, July 2007, Vol. 48:7, pp. 1069-1076).
Marketing strategy
Positron currently is establishing marketing and distribution channels, with cardiology offices as "the primary target," Oliverio said. "However, I believe the largest societal benefit of this technology will be through placing these systems within the rural hospital market."
Positron also created a program called PosiRx to give imaging providers the choice of radiopharmaceuticals, dispensing systems, molecular imaging devices, and equipment service directly from the company. Positron will offer distribution and dose dispensing of radiopharmaceuticals through its Nuclear Pharm-Assist device.
With the U.S. economy currently in dire straits, limiting the ability of many hospitals to pursue new capital equipment purchases, Positron also is looking to partner with a financing company to stimulate the PET market.
"With cardiac PET, it is known that it will have a clinical benefit if it is put into practice," Oliverio said. "At the same time, the ability to monitor therapy and take advantage of the trends of medicine going toward proactive, nonsurgical methods, and the ability to track coronary disease progression, reversal, or cessation of disease, is priceless, in my opinion."
By Wayne Forrest
AuntMinnie.com staff writer
March 4, 2009
Related Reading
Neusoft Positron files 510(k) for Attrius, January 29, 2009
Positron launches cardiac nuclear medicine package, January 9, 2009
Positron hires medical director, December 17, 2008
Neusoft taps TUV for FDA help, October 23, 2007
Positron opens Chinese joint venture, February 13, 2006
Copyright © 2009 AuntMinnie.com




















