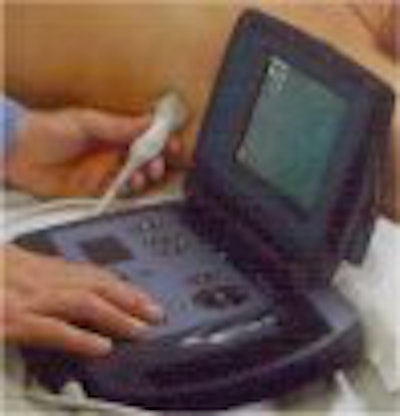
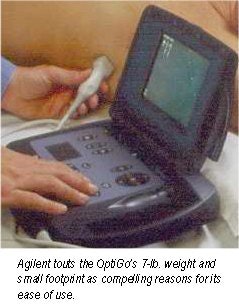
The portable ultrasound market heated up this week with the introduction of a handheld ultrasound scanner by Agilent Technologies at the American College of Cardiology meeting in Orlando, FL. The Andover, MA-based manufacturer is putting its OptiGo point-of-care ultrasound device onto a field developed by players such as Terason and SonoSite.
Agilent representatives noted that ultrasound can be performed at modest costs, compared to other imaging modalities such as MRI and CT, or other diagnostic practices such as angiography. Ultrasound is also accepted by clinicians, is completely noninvasive, and causes little inconvenience for patients.
Company officials point to the acceptance of portable devices in the clinical community, such as personal digital assistants, as proof of OptiGo's concept. In addition, cardiac clinicians have long advocated the use of echocardiograms as part of a routine cardiac exam.
"I believe we are on the threshold of a day in which the echocardiogram truly becomes an extension of the physical examination and provides a 'window on the heart' that is unmatched by the physical examination alone," said Dr. Richard Popp in the January 1998 ACC Current Journal Review (Volume 7:1, pp. 79-81).
The goal behind the design of the portable unit was to provide a device that could be used as easily as a stethoscope. By providing cardiac ultrasound capability at the point of care, cardiologists will no longer have to perform the extra steps for ordering basic ultrasound testing, which can increase patient anxiety, delays, and costs.
OptiGo is a color-flow Doppler ultrasound system with a 2.5-MHz phased-array transducer for all digital 2-D imaging. Images can be viewed in real-time during the exam, and still-frame images can be stored to a memory card for printing, e-mailing, or archiving. OptiGo also features a small footprint, is lightweight, and operates on either battery or A/C power. As debuted, the unit has a street price of under $12,000.
 |
The device was designed for clinical evaluation of left ventricular function, valve function, chamber size, and pericardial effusion. Agilent representatives foresee product placement in physicians' offices, cardiac care units, intensive care units, emergency rooms, and outreach clinics, as well as for use during hospital rounds.
By Jonathan S. Batchelor
AuntMinnie.com staff writer
March 21, 2001
Related Reading
Philips, Agilent deal hits U.S. snag, February 2, 2001
Agilent unveils new multispecialty ultrasound scanner, November 26, 2000
Philips plans $1.7 billion acquisition of Agilent healthcare group, November 17, 2000
Agilent plans restructuring, layoffs, August 14, 2000
Agilent to distribute Cedara software in Asia Pacific, May 15, 2000
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com